ƒøƒ⁄»ð
œ¬¡–“∫ÃÂæ˘¥¶”⁄25 °Ê£¨”–πÿ– ˆ’˝»∑µƒ «
| A£Æƒ≥ŒÔ÷ »Ð“∫µƒpH>7£¨‘Ú∏√ŒÔ÷ “ª∂® «ºÓªÚ«øºÓ»ıÀ·—Œ |
| B£ÆpH£Ω6.5µƒ≈£ƒÃ÷–c(H£´) «pH£Ω4.5µƒH2SO4»Ð“∫÷–c(H£´)µƒ100±∂ |
| C£ÆpH£Ω3µƒ¥◊À·”ÎpH£Ω11µƒNaOH»Ð“∫µ»Ãª˝ªÏ∫œ∫ۻГ∫÷–£∫ c(CH3COO£≠)>c(Na£´)>c(H£´)>c(OH£≠) |
| D£ÆAgCl‘⁄µ»≈®∂»µƒCaCl2»Ð“∫∫ÕNaCl»Ð“∫÷–µƒ»ÐΩ‚∂»œýÕ¨ |
C
Ω‚Œˆ

œ¬¡–Àµ∑®≤ª’˝»∑µƒ «
| A£ÆNaHCO3∫ÕNa2CO3ªÏ∫œ»Ð“∫÷–£∫c(Na£´)£´c(H£´)£Ωc(OH£≠)£´c(HCO3-£©£´2c(CO32-) |
| B£Æ≥£Œ¬œ¬£¨≈®∂»æ˘Œ™0£Æ1 mol°§L£≠1œ¬¡–∏˜»Ð“∫µƒpH£∫NaOH>Na2CO3> NaHCO3> NH4Cl |
| C£ÆœÚ±˘¥◊À·÷–÷µŒº”ÀÆ£¨¥◊À·µƒµÁ¿Î≥Ã∂»°¢pHæ˘œ»‘ˆ¥Û∫Ûºı–° |
| D£Æ≥£Œ¬œ¬£¨pH=1µƒœ°¡ÚÀ·”Î¥◊À·»Ð“∫÷–£¨c(SO42-)”Îc(CH3COO£≠)÷Ʊ»Œ™2°√1 |
‘⁄18 °Ê ±£¨H2SO3µƒK1£Ω1£Æ5°¡10-2°¢K2£Ω5£Æ0°¡10-7£¨H2CO3µƒK1£Ω1£Æ4°¡10-7°¢K2£Ω2£Æ7°¡10-11£¨‘Úœ¬¡–Àµ∑®÷–’˝»∑µƒ « ( )
| A£Æ∂ý‘™»ıÀ·µƒÀ·–‘÷˜“™”…µ⁄“ª≤ΩµÁ¿Îæˆ∂®£¨—«¡ÚÀ·µƒÀ·–‘»ı”⁄úÀ· |
| B£Æ∂ý‘™»ıÀ·µƒÀ·–‘÷˜“™”…µ⁄∂˛≤ΩµÁ¿Îæˆ∂®£¨ÃºÀ·µƒÀ·–‘»ı”⁄—«¡ÚÀ· |
| C£Æ∂ý‘™»ıÀ·µƒÀ·–‘÷˜“™”…µ⁄“ª≤ΩµÁ¿Îæˆ∂®£¨—«¡ÚÀ·µƒÀ·–‘«ø”⁄úÀ· |
| D£Æ∂ý‘™»ıÀ·µƒÀ·–‘÷˜“™”…µ⁄∂˛≤ΩµÁ¿Îæˆ∂®£¨ÃºÀ·µƒÀ·–‘«ø”⁄—«¡ÚÀ· |
≥£Œ¬œ¬£¨œÚ20 mL 0.2 mol/L H2A»Ð“∫÷–µŒº”0.2 mol/L NaOH»Ð“∫°£”–πÿ¡£◊”µƒŒÔ÷ µƒ¡ø±‰ªØ»ÁÕº(∆‰÷–¢Ò¥˙±ÌH2A£¨¢Ú¥˙±ÌHA£≠£¨¢Û¥˙±ÌA2£≠)£¨∏˘æðÕº æ≈–∂œ£¨œ¬¡–Àµ∑®’˝»∑µƒ «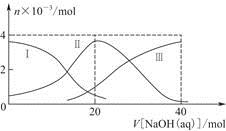
| A£Æµ±V[NaOH(aq)]£Ω20 mL ±£¨»Ð“∫÷–¿Î◊”≈®∂»¥Û–°πÿœµ£∫ c(Na£´)>c(HA£≠)>c(H£´)>c(A2£≠)>c(OH£≠) |
| B£Æµ»Ãª˝µ»≈®∂»µƒNaOH»Ð“∫”ÎH2A»Ð“∫ªÏ∫œ∫Û£¨∆‰»Ð“∫÷–ÀƵƒµÁ¿Î≥Ã∂»±»¥øÀÆ¥Û |
| C£ÆH2Aµ⁄“ª≤ΩµÁ¿Îµƒ∑Ω≥Ã ΩŒ™H2A=HA£≠£´H£´ |
| D£ÆœÚNaHA»Ð“∫º”»ÎÀÆœ° Õµƒπ˝≥Ã÷–£¨pHø…ƒÐ‘ˆ¥Û“≤ø…ƒÐºı–° |
“—÷™H2CO3µƒÀ·–‘«ø”⁄H2S£¨Ω´a mol°§L£≠1NaHS”Îb mol°§L£≠1NaOH¡Ω÷÷œ°»Ð“∫µ»Ãª˝ªÏ∫œ(a>0£¨b>0)£¨À˘µ√»Ð“∫÷–Œ¢¡£º‰µƒŒÔ÷ µƒ¡ø≈®∂»πÿœµ’˝»∑µƒ «
| A£Æa£Ωb ±£∫c(OH£≠)£Ωc(H£´)£´c(HS£≠) |
| B£Æa£Ω2b ±£∫c(S2£≠)>c(HS£≠)>c(OH£≠)>c(H£´) |
| C£Æa£Ω3b ±£∫c(Na£´)£´c(H£´)£Ω2c(S2£≠)£´c(HS£≠)£´c(OH£≠) |
| D£Æa£Ω4b ±£∫4c(Na£´)£Ω5c(S2£≠)£´5c(HS£≠)£´5c(H2S) |
25°Ê ±£¨≈®∂»æ˘Œ™1 mol/LµƒAX°¢BX°¢AY°¢BYÀƒ÷÷’˝—Œ»Ð“∫£¨AX»Ð“∫µƒpH£Ω7«“»Ð“∫÷–c(X£≠)£Ω1 mol/L£¨BX»Ð“∫µƒpH£Ω4£¨BY»Ð“∫µƒpH£Ω6°£œ¬¡–Àµ∑®’˝»∑µƒ «
| A£ÆAY»Ð“∫µƒpH–°”⁄7 |
| B£ÆAY»Ð“∫µƒpH–°”⁄BY»Ð“∫µƒpH |
| C£Æœ° ÕœýÕ¨±∂ ˝£¨BX»Ð“∫µƒpH±‰ªØ–°”⁄BY»Ð“∫ |
| D£ÆµÁ¿Î∆Ω∫‚≥£ ˝K(BOH)–°”⁄K(HY) |
ƒ≥ºÓ–‘»Ð“∫÷–÷ª∫¨”–Na£´°¢CH3COO£≠°¢H£´°¢OH£≠ 4÷÷¿Î◊”°£œ¬¡–√Ë ˆ’˝»∑µƒ «
| A£Æ∏√»Ð“∫“ª∂® «”…µ»ŒÔ÷ µƒ¡ø≈®∂»°¢µ»Ãª˝µƒNaOH»Ð“∫∫ÕCH3COOH»Ð“∫ªÏ∫œ∂¯≥… |
| B£Æ∏√»Ð“∫“ª∂®”…pH£Ω3µƒCH3COOH»Ð“∫”ÎpH£Ω11µƒNaOH»Ð“∫µ»Ãª˝ªÏ∫œ∂¯≥… |
| C£Æ∏√»Ð“∫÷–¿Î◊”≈®∂»“ª∂®Œ™c(Na£´)>c(CH3COO£≠)>c(OH£≠)>c(H£´) |
| D£Æº”»Î“ª∂®¡ø±˘¥◊À·£¨c(CH3COO£≠)ø…ƒÐ¥Û”⁄°¢µ»”⁄ªÚ–°”⁄c(Na£´) |
≥£Œ¬œ¬£¨º∏÷÷ƒ—»ÐµÁΩ‚÷ µƒ»Ð∂»ª˝∫Õ»ıÀ·µƒµÁ¿Î≥£ ˝»Áœ¬±ÌÀ˘ æ£∫
‘Úœ¬¡–Àµ∑®≤ª’˝»∑µƒ «
| A£ÆœýÕ¨Œ¬∂»°¢œýÕ¨≈®∂»µƒƒ∆—Œ»Ð“∫µƒpH£∫Na2S£æNa2CO3£æNaHS£æNaCl£æNaHSO4 |
| B£Æ‘⁄NaHS»Ð“∫÷–µŒº”¡ÚÀ·Õ≠»Ð“∫£¨…˙≥…∫⁄…´≥¡µÌ£∫HS£≠£´Cu2£´=CuS°˝£´H£´ |
| C£Æ≥˝»•π¯¬Ø÷–µƒÀÆπ∏ ±£¨Õ®≥£œ»º”»Î◊„¡ø¡ÚÀ·ƒ∆»Ð“∫£¨Ω´ÃºÀ·∏∆◊™ªØ≥…¡ÚÀ·∏∆£¨»ª∫Û‘Ÿ”√À·“∫¥¶¿Ì |
D£Æ‘⁄Mg(HCO3)2»Ð“∫÷–µŒº”≥Œ«Â ت“ÀÆ∑¢…˙∑¥”¶µƒ¿Î◊”∑Ω≥Ã ΩŒ™Mg2£´£´2 £´2Ca2£´£´4OH£≠=Mg(OH)2°˝£´2CaCO3°˝£´2H2O £´2Ca2£´£´4OH£≠=Mg(OH)2°˝£´2CaCO3°˝£´2H2O |
”–ê˝œýÕ¨°¢pHœýµ»µƒ…’ºÓ»Ð“∫∫Õ∞±ÀÆ£¨œ¬¡–– ˆ÷–’˝»∑µƒ «(°°°°)
| A£Æ¡Ω»Ð“∫ŒÔ÷ µƒ¡ø≈®∂»œýÕ¨ |
| B£Æ”√Õ¨≈®∂»µƒ—ŒÀ·÷–∫Õ ±£¨œ˚∫ƒ—ŒÀ·µƒÃª˝œýÕ¨ |
| C£Æ¡Ω»Ð“∫÷–OH£≠≈®∂»œýÕ¨ |
| D£Æº”»Îµ»Ãª˝µƒÀÆœ° Õ∫Û£¨pH»‘œýµ» |