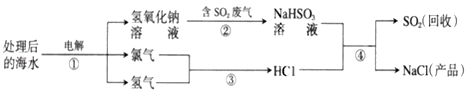
��Ŀ����
����Ŀ������˵����ȷ����
A. ��֪C2H4(g)+H2(g)=C2H6(g)��H=-137.0kJ/mol,����ϩ�������ӳ�ʱ�ų�68.5 kJ��������Ӧ�����б��ƻ���̼ԭ��֮��Ĺ��õ��Ӷ���ĿΪNA
B. ij�¶���CaC2O4 ��Ksp=2.5��10-9����0.02mol/L����ʯ��ˮ��0.01mol/LH2C2O4��Һ�������ϣ�������Һ��C2042-�����ʵ���Ũ��Ϊ5��10-7mol/L
C. ij�¶��£�һ��Ũ��CH3COOH��Һ��ˮϡ�ͺ���Һ�� ��ֵ������ƽ�ⳣ������
��ֵ������ƽ�ⳣ������
D. CH3COONa��CaCl2�����Һ:c(Na+)+c(Ca2+)=c(CH3C00-)+c(CH3COOH)+2c(Cl-)
���𰸡�B
��������C2H4(g)+H2(g)=C2H6(g)��H=-137.0kJ/mol,����ϩ�������ӳ�ʱ�ų�68.5 kJ������˵���μӷ�Ӧ����ϩ�����ʵ�����0.5mol����Ӧ�����б��ƻ���̼ԭ��֮��Ĺ��õ��Ӷ���ĿΪ0.5NA����A������0.02mol/L����ʯ��ˮ��0.01mol/LH2C2O4��Һ�������ϣ���Ӧ��Ca2+��Ũ����0.005mol/L��C2042-�����ʵ���Ũ��Ϊ![]() ����B��ȷ��ij�¶��£�һ��Ũ��CH3COOH��Һ��ˮϡ�ͺ�������ƽ�������ƶ�����Һ��
����B��ȷ��ij�¶��£�һ��Ũ��CH3COOH��Һ��ˮϡ�ͺ�������ƽ�������ƶ�����Һ��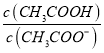 ��ֵ��С������ƽ�ⳣ����������C���������������غ㣬CH3COONa��CaCl2�����Һ����c(Na+)+2c(Ca2+)=c(CH3C00-)+c(CH3COOH)+ c(Cl-)����D������
��ֵ��С������ƽ�ⳣ����������C���������������غ㣬CH3COONa��CaCl2�����Һ����c(Na+)+2c(Ca2+)=c(CH3C00-)+c(CH3COOH)+ c(Cl-)����D������

 ��У����ϵ�д�
��У����ϵ�д�