��Ŀ����
5����ͼ��ʾ������ƿ�зֱ�װ�뺬��̪��0.01mol/L CH3COONa��Һ�����ֱ������ʢ��ˮ���ձ��У�Ȼ�����ձ����м�����ʯ�ң����ձ����м���NH4NO3���壬�ձ����в����κ����ʣ�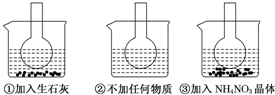
��1������̪��0.01mol/L CH3COONa��Һ��dz��ɫ��ԭ��ΪCH3COO-+H2O?CH3COOH+OH-��ʹ��Һ�Լ��ԣ����Է�̪�Ժ�ɫ��
��2��ʵ������з�����ƿ������Һ��ɫ�����ƿ������Һ��ɫ��dz��������������ȷ����BD��
A��ˮ�ⷴӦΪ���ȷ�Ӧ
B��ˮ�ⷴӦΪ���ȷ�Ӧ
C��NH4NO3����ˮʱ�ų�����
D��NH4NO3����ˮʱ��������
��3����0.01mol/L CH3COONa��Һ�зֱ��������Ũ���ᡢNaOH���塢Na2CO3���塢FeSO4���壬ʹCH3COO-ˮ��ƽ���ƶ��ķ���ֱ�Ϊ�ҡ������ң�������ҡ����ƶ�������
���� ��1��CH3COONaΪǿ�������Σ����������ˮ���ʹ��Һ�ʼ��ԣ�
��2��CaO��ˮ��Ӧ�Ƿ��ȷ�Ӧ�����Իᵼ����Һ�¶����ߣ�������ܽ���������������ᵼ����Һ�¶Ƚ��ͣ������¶ȴٽ�CH3COONaˮ�⣬�����¶�����CH3COONaˮ�⣻
��3��CH3COO-+H2O?CH3COOH+OH-����������������ӷ�Ӧ�����ʴٽ�ˮ�⡢���뺬�����������ӵ���������ˮ�⣬�ݴ˷������
��� �⣺��1��CH3COONaΪǿ�������Σ����������ˮ���ʹ��Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪCH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��ʹ��Һ�Լ��ԣ����Է�̪�Ժ�ɫ��
��2��CaO+H2O=Ca��OH��2���÷�Ӧ�Ƿ��ȷ�Ӧ�����Իᵼ����Һ�¶����ߣ�������ܽ���������������ᵼ����Һ�¶Ƚ��ͣ������¶ȴٽ�CH3COONaˮ�⣬�����¶�����CH3COONaˮ�⣬����CH3COONaˮ�ⷴӦΪ���ȷ�Ӧ���ʴ�Ϊ��BD��
��3��CH3COO-+H2O?CH3COOH+OH-����������Ũ���ᡢFeSO4����ʱ�������Ӻ��������Ӷ������������ӷ�Ӧ�����Դٽ�ˮ�⣬ƽ�������ƶ�����������NaOH���塢Na2CO3���壬����������Ũ���������ƴ�����ˮ�⣬ƽ�����淴Ӧ�����ƶ����ʴ�Ϊ���ң������ң�
���� ���⿼��������ˮ�⣬��ȷ����ˮ��ԭ���ǽⱾ��ؼ���֪��Ӱ������ˮ������أ�����ˮ�ⷢ��ƽ���ƶ�ʱˮ��ƽ�ⳣ����һ���仯����Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ���Խ�����KMnO4����H2O2��2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O | |
| B�� | ����SO2ͨ��Ca��ClO��2��Һ�У�SO2+H2O+Ca2++2ClO-�TCaSO3��+2HClO | |
| C�� | ������Һ����ϴ���ۣ�CO32-+2H2O?H2CO3+2OH- | |
| D�� | �����������ۼ���ϡ�����У�Fe+4H++NO3-�TFe3++NO��+2H2O |
| A�� | Cd | B�� | NiO��OH�� | C�� | Cd��OH��2 | D�� | Ni��OH��2 |
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �ڢ� |
| A�� | 0.4mol | B�� | 0.2mol | C�� | 0.2mol��x��0.4mol | D�� | ��0.2mol |
| A�� | þ�����ᷴӦ��2Fe+6H+�T2Fe3++3H2�� | |
| B�� | �Ȼ�����Һ�м���ͭƬ��Fe3++Cu�TFe2++Cu2+ | |
| C�� | ����ͨ��ˮ�У�CI2+H2O�T2H++CI-+CIO- | |
| D�� | ������Һ�м�������������Һ��2H++SO42-+Ba2++2OH-�TBaSO4��+2H2O |
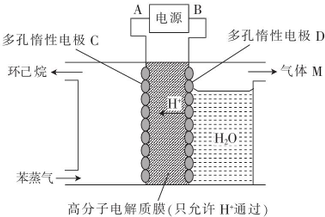 ���ܵ��о��������ǵ����ѧ�о����ȵ�֮һ����Ѱ��������Խ����ȫ�Ըߡ��۸�����������Ĵ���
���ܵ��о��������ǵ����ѧ�о����ȵ�֮һ����Ѱ��������Խ����ȫ�Ըߡ��۸�����������Ĵ���