��Ŀ����
��12�֣�����������һ����Ҫ�Ļ�ѧ��Ʒ���ڹ�ũҵ�������ճ����������Ź㷺Ӧ�ã���ҵ���������������
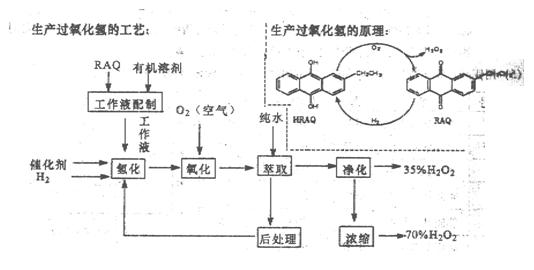
����������������ԭ�����գ��ش��������⣺
��1�����������������ԭ���У�ԭ��������Ϊ ��
��2���ù������л��ܼ�ΪҺ̬���������࣬�ܷ����Ҵ����棿
��3��Ϊ����߾���Ч�棬�ù����⻯��Ӧ�����˼Ӵ����⣬���ɲ�ȡ�Ĵ�ʩ�� ��
A���ʵ����� B����ѹ C��������Һ���л��ܼ�����
��4���ù����С���������Ŀ���� ��
��5���ù�������35%��H2O2�õ�70%��H2O2�ɲ�ȡ���������� ��
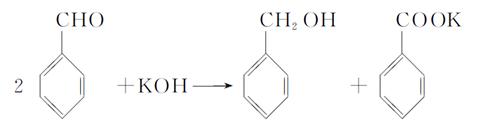
��6�������ữ�Ĺ���������Һ���ܽ����ͭ����д���÷�Ӧ�����ӷ���ʽΪ ��

����������������ԭ�����գ��ش��������⣺
��1�����������������ԭ���У�ԭ��������Ϊ ��
��2���ù������л��ܼ�ΪҺ̬���������࣬�ܷ����Ҵ����棿
��3��Ϊ����߾���Ч�棬�ù����⻯��Ӧ�����˼Ӵ����⣬���ɲ�ȡ�Ĵ�ʩ�� ��
A���ʵ����� B����ѹ C��������Һ���л��ܼ�����
��4���ù����С���������Ŀ���� ��
��5���ù�������35%��H2O2�õ�70%��H2O2�ɲ�ȡ���������� ��
��6�������ữ�Ĺ���������Һ���ܽ����ͭ����д���÷�Ӧ�����ӷ���ʽΪ ��
ÿ�վ�2��
��1��100%
��2������Ϊ�Ҵ������������Һ��������� �ȶ����ܣ�����ȡ�������빤��Һ���루�ж���ȷ���ɵ�2�֣�
�ȶ����ܣ�����ȡ�������빤��Һ���루�ж���ȷ���ɵ�2�֣�
��3��AB����ѡ���÷֣�©ѡ��1�֣�
��4������RAQ����HRAQ�����л��ܼ���ѭ��ʹ�á�
��5����ѹ����������1�֣�
��6��Cu+2H++H2O2=Cu2++2H2O
��1��100%
��2������Ϊ�Ҵ������������Һ���������
 �ȶ����ܣ�����ȡ�������빤��Һ���루�ж���ȷ���ɵ�2�֣�
�ȶ����ܣ�����ȡ�������빤��Һ���루�ж���ȷ���ɵ�2�֣���3��AB����ѡ���÷֣�©ѡ��1�֣�
��4������RAQ����HRAQ�����л��ܼ���ѭ��ʹ�á�
��5����ѹ����������1�֣�
��6��Cu+2H++H2O2=Cu2++2H2O
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
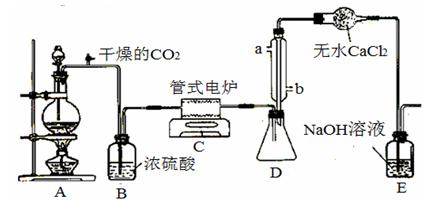
 ��CO32-��OH
��CO32-��OH �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩.
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩. L-1 H2SO4 b��0.01mol
L-1 H2SO4 b��0.01mol Ԥ������ͽ���
Ԥ������ͽ���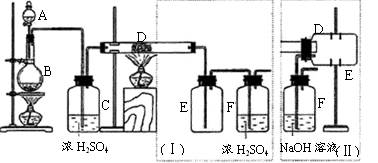
 ���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L��
���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L��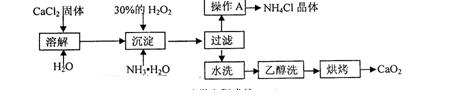
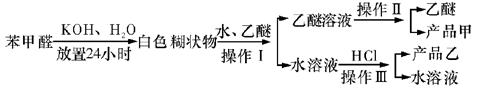
 ������ţ���
������ţ��� ��
��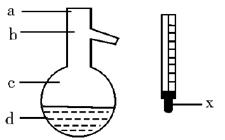
 ����ȡ������װ����ͼI�͢�
����ȡ������װ����ͼI�͢� ��
��