��Ŀ����
��֪����CH3OH(g)��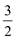 O2(g)=CO2(g)��2H2O(g) ��H����a kJ��mol
O2(g)=CO2(g)��2H2O(g) ��H����a kJ��mol
��CO(g)��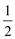 O2(g)=CO2(g) ��H����b kJ��mol
O2(g)=CO2(g) ��H����b kJ��mol
��H2(g)��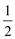 O2(g)=H2O(g) ��H����c kJ��mol
O2(g)=H2O(g) ��H����c kJ��mol
��H2(g)��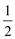 O2(g)=H2O(l) ��H����d kJ��mol
O2(g)=H2O(l) ��H����d kJ��mol
������������ȷ����
A. �������Ȼ�ѧ����ʽ��֪d��c
B. H2��ȼ����Ϊd kJ��mol
C. CH3OH(g)=CO(g)��2H2(g) ��H��(b��2c��a)kJ��mol
D. ��CO��H2�����ʵ���֮��Ϊ1��2ʱ������ȫȼ������CO2��H2O(l)ʱ���ų�Q kJ��������������CO�����ʵ���Ϊ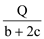 mol
mol
Ԫ��X��Z��Ԫ�����ڱ���4������Ԫ�أ������ʻ�ṹ��Ϣ���±����й�����Ԫ�ص�����������ǣ�������
Ԫ�� | X | Y | W | Z |
�����Ϣ | �����ᴦ�������ʱ���ɵ�ͨ��״����Ϊ�Ϻ�ɫ�Ĺ��壬���Ƽ������������� | ��ҵ���ڱ���ʯ���ڵ������£��õ�ⷨ��ȡ�䵥�ʣ� | ��������������������ȡʳ�κ����ˮĸҺ�з��ֵģ�������������Ԫ�ء��� | ԭ�ӵĵ���������������������6�� |
A. ʵ���ҿ��ں���ҵĽ���Һ�еμ�˫��ˮ����ȡԪ��X�ĵ���
B. Ԫ��X��Y�ĵ��ʳ�ֻ�Ϻ�μ�����ˮ���ɿ���������ɫ�������ɣ�˵���÷�Ӧ��ų���������
C. Ԫ��X����̬�⻯��ķе����Ԫ��W����̬�⻯��ķе�
D. Ԫ��Z��һ�ֽϻ��õĽ���Ԫ�أ��������ȷ���Z�����������Ʊ�Z�ĵ���


 aOH��Һ���ԭ��أ��为����ӦʽΪ��
aOH��Һ���ԭ��أ��为����ӦʽΪ��
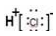 B. �÷�Ӧ�ǹ�ҵ��ȡ����Ļ�ѧ��Ӧԭ��
B. �÷�Ӧ�ǹ�ҵ��ȡ����Ļ�ѧ��Ӧԭ��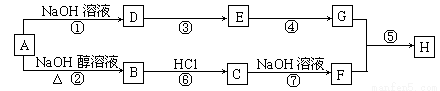
 2NaCl+NaClO3
2NaCl+NaClO3