��Ŀ����
ˮ����(N2H4��H2O)����ɫ����ǿ��ԭ�Ե�Һ�壬ʵ�����Ʊ�ˮ���µ�ԭ��Ϊ��
CO(NH2)2+2NaOH+NaClO==Na2CO3+N2H4��H2O+NaCl
�ݴˣ�ijѧ�����������ʵ��.
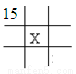
���Ʊ�NaClO��Һ��ʵ��װ������ͼ��ͼ��ʾ�����ּг�װ����ʡ�ԣ�
��֪��3NaClO 2NaCl+NaClO3
2NaCl+NaClO3

��1������30 %NaOH��Һʱ�����貣����������Ͳ���__________������ĸ����
A.����ƿ B���ձ� C����Һ�� D.������
��2��װ��I�з��������ӷ�Ӧ����ʽ��_______________�����в�����a������Ϊ____________��Ϊ�����NaClO�IJ��ʣ����I�в�����Cl2���о����������Լ���________________�������ñ�ˮԡ�����¶���30�����£�����ҪĿ��___________________
����ȡˮ���¡�ʵ��װ������ͼ��ͼ��ʾ
��3����Ӧ�����У������Һ©������Һ�ĵ��ٹ��죬 ����N2 H4��H2O����A�з�Ӧ������������������Ʒ������˽��ͣ���д�����Ͳ��ʵ���ػ�ѧ��Ӧ����ʽ____________________����ַ�Ӧ��������A�ڵ���Һ���ɵõ�ˮ���µĴֲ�Ʒ��Bװ�õ�������______________
���ⶨ�µĺ�����
��4����ȡ���0.3000 g����ˮ���20.0 mL��Һ��һ����������0.1500 mol��L-1 ��I2��Һ�ζ���
��֪: N2H4��H2O + 2I2 = N2��+ 4HI + H2O��
�ٵζ�ʱ������ѡ�õ�ָʾ��Ϊ____________;
��ʵ��������I2��Һ��ƽ��ֵΪ20. 00 mL�������N2H4��H2O����������Ϊ_____________��
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д��±���Ԫ�����ڱ�ʾ��ͼ��һ���֣�����A~I��Ԫ�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | A | |||||||
2 | D | E | G | I | ||||
3 | B | C | F | H |
(1)����Ԫ�ػ�ѧ��������õ���_______��ֻ�и����ϼ۶��������ϼ۵���_______����������ǿ�ĵ�����_______����ԭ����ǿ�ĵ�����_______��
(2)����Ԫ������������ˮ����ļ�����ǿ�Ļ�������__________��������ǿ�Ļ�������__________�������ԵĻ�������________��
(3)A�ֱ���D��E��F��G��H�γɵĻ����������ȶ�����________��
(4)��B��C��D��E��F��G��H�У�ԭ�Ӱ뾶������________��


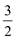 O2(g)=CO2(g)��2H2O(g) ��H����a kJ��mol
O2(g)=CO2(g)��2H2O(g) ��H����a kJ��mol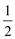 O2(g)=CO2(g) ��H����b kJ��mol
O2(g)=CO2(g) ��H����b kJ��mol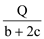 mol
mol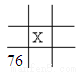 B.
B. 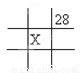 C.
C.  D.
D.