��Ŀ����
��10�֣�
�������зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
����д���пհף�
��1����������պ���ʱ������Ҫ���Ǽ��⣬����Ҫ�õ���������
��������������ѡ��������������ñ����ĸ��д�ڿհ״���
| A���ձ� | B������ | C�������� | D�������� E���ƾ��� F�������� |
��3��������У�ijѧ��ѡ���ñ�����ȡ���������
��
��4�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ���
��
��1��B��D��E
��2�����ˡ�����
��3������ˮ�����ܣ����ڱ��е��ܽ�����Ա���ˮ�д�
��4����С�Թ�ȡ������Һ��������²㣨ˮ�㣩��Һ�����뼸�ε�����Һ�������ɫ֤�������ⵥ��
����

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ
 ������������Ԫ��֮һ������ֲ���纣���������к��зḻ���Ե�������ʽ���ڵĵ�Ԫ�أ������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
������������Ԫ��֮һ������ֲ���纣���������к��зḻ���Ե�������ʽ���ڵĵ�Ԫ�أ������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
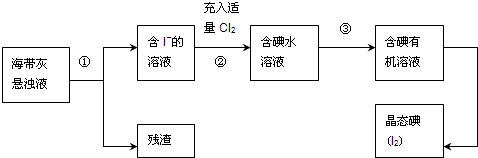
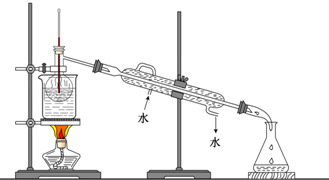

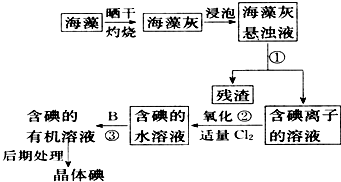 ����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ�������ӵ���ʽ���ڣ�ʵ���дӺ�����ȡ���������ͼ��ʾ��
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ�������ӵ���ʽ���ڣ�ʵ���дӺ�����ȡ���������ͼ��ʾ��