جâؤ؟ؤعبف
¢ٌ.¹¤زµةدسأآءحء؟َضئب،آءµؤء÷³جبçدآ£؛

اë»ط´ًدآءذختجâ£؛
(1)²ظ×÷¢ٌ،¢²ظ×÷¢ٍ،¢²ظ×÷¢َ¶¼سأµ½µؤ²£ء§زائ÷سذ__________________________،£
(2)ذ´³ِسةبـز؛Bةْ³ةAl(OH)3µؤہë×س·½³جت½£؛____________________________،£
(3)¹¤زصء÷³جضذةو¼°رُ»¯»¹ش·´س¦µؤ»¯ر§·½³جت½خھ£؛_________________________،£
(4)ةْ²ْ¹³جضذ£¬³NaOH،¢H2O؟ةزشر»·ت¹سأح⣬»¹؟ةزشر»·ت¹سأµؤخïضتسذ________(جر§ت½)،£سأ´ث·¨ضئب،آءµؤ¸±²ْئ·تا________(جر§ت½)،£
¢ٍ.خز¹ْ¸كآ¯ةْ²ْ¸÷·½أوب،µأءثدشضّ½ّ²½£¬µ«شع×تش´؛حؤـش´ہûسأآت،¢¸كآ¯´َذح»¯،¢جل¸ك²ْزµ¼¯ضذ¶بزش¼°»·±£µب·½أو»¹¸ْ¹ْ¼ت´وشع؛ـ´َ²î¾à£¬سذ´½ّز»²½جل¸ك£¬إ¬ء¦دٍ¸ضجْا؟¹ْآُ½ّ،£اë»ط´ًدآءذختجâ£؛
(1)¸كآ¯ء¶جْµؤشءدسذجْ؟َ،¢½¹ج؟؛حت¯»زت¯£¬ئنضذئًبغ¼ء×÷سأµؤتا________£¬ؤ؟µؤتا³ب¥جْ؟َت¯ضذµؤآِت¯£¬ئن²ْخïأـ¶ب±بجْ________£¬ثùزششعجْث®µؤ________(جî،°ةد²؟،±»ٍ،°دآ²؟،±)ذخ³ةآ¯شü¶ّسëجْث®·ضہë،£
(2)½¹ج؟شع¸كآ¯ء¶جْضذئً×إ¾ظ×مالضطµؤ×÷سأ£¬دآءذ²»تôسع½¹ج؟×÷سأµؤتا________،£
A£®×÷خھب¼ءد£¬خھء¶جْضذµؤ»¯ر§·´س¦جل¹©ؤـء؟
B£®×÷خھ»¹ش¼ءسë¶رُ»¯ج¼·´س¦²ْةْ»¹شرُ»¯جْµؤز»رُ»¯ج¼
C£®¶ش¸كآ¯ضذµؤخïءدئًµ½ض§³إ؛حتèة¢µؤ×÷سأ
D£®×÷خھبـ¼ء£¬³ب¥جْ؟َت¯ضذµؤشسضت
(3)¸كآ¯ء¶جْµؤخغب¾·ا³£ردضط£¬ؤ؟ا°خز¹ْ²؟·ض´َ³اتذضذµؤ¸ضجْ³§½è×إ°لا¨µؤ»ْ»لز²شع½ّذذ×إ¹¤زص¸ؤ½ّ،£¸كآ¯ء¶جْµ¼ضآµؤ»·¾³خغب¾سذ________،£
A£®³ôرُ؟ص¶´ B£®ثلسê C£®ز»رُ»¯ج¼¶¾؛¦ D£®ة³³¾±©
(4)ذ´³ِ¸كآ¯ء¶جْضذسëج¼شھثطسذ¹طµؤرُ»¯»¹ش·´س¦»¯ر§·½³جت½£؛____________________________،£
(5)´س،°ب·د،±ہûسأ،¢»·¾³±£»¤µب½ا¶ب؟¼آا£¬¸ضجْئَزµشعةْ²ْضذس¦²ةب،µؤز»ذ©´ëت©سذ(¾ظ³ِ2ضض´ëت©¼´؟ة) _______________________________________،£
¢ٌ.(1)ةص±،¢آ©¶·،¢²£ء§°ô
(2)2AlO2،ھ£«CO2£«3H2O=2Al(OH)3،£«CO32،ھ
(3)2Al2O3(بغبع) 4Al£«3O2،ü
4Al£«3O2،ü
(4)CaO؛حCO2،،Fe2O3؛حO2
¢ٍ.(1)ت¯»زت¯،،ذ،،،ةد²؟،،(2)D،،(3)BC
(4)C£«O2 CO2£»CO2£«C
CO2£»CO2£«C 2CO£»Fe2O3£«3CO
2CO£»Fe2O3£«3CO 2Fe£«3CO2
2Fe£«3CO2
(5)سأء¶جْ³§µؤآ¯شü(»ٍCaSiO3)×÷خھث®ؤ೧µؤشءد£»سأ·¢µç³§µؤأ؛ي·ت¯؛ح·غأ؛»ز×÷خھث®ؤ೧µؤشءد£»½«ت¯»زت¯ىرةص³ةةْت¯»ز£¬سأسعخüتص·¢µç³§؛ح½¹»¯³§ب¼أ؛ت±²ْةْµؤSO2£¬¼ُةظ¶ش؟صئّµؤخغب¾£»½¨ء¢خغث®´¦ہيدµح³(بخر،ء½جُ£¬ئنثû؛دہي´ً°¸ز²؟ةزش)
،¾½âخِ،؟¢ٌ.(1)²ظ×÷¢ٌ،¢²ظ×÷¢ٍ،¢²ظ×÷¢َ¶¼تا¹آث£¬¹آثذèزھةص±،¢آ©¶·،¢²£ء§°ô،£(2)NaAlO2بـز؛ضذح¨بëCO2ةْ³ةAl(OH)3³ءµي؛حNa2CO3£¬ئنہë×س·½³جت½خھ2AlO2،ھ£«CO2£«3H2O=2Al(OH)3،£«CO32،ھ،£(3)ض»سذµç½âبغبعAl2O3µؤ·´س¦تôسعرُ»¯»¹ش·´س¦،£(4)µأµ½µؤCaCO3تـبب·ض½âµأµ½CaO،¢CO2£¬؟ة¼ûCaO،¢CO2؟ةر»·ت¹سأ،£²ظ×÷¢ٌثùµأµؤFe2O3؛حµç½âبغبعAl2O3µأµ½µؤO2خھ¸±²ْئ·،£
¢ٍ.(1)¸كآ¯ء¶جْµؤشءدضذ±طذëسذ؛دتت±بہµؤت¯»زت¯£¬ئن×÷سأتا×÷خھشسضتµؤبـ¼ء£¬ؤ؟µؤ¾حتا³ب¥جْ؟َت¯ضذµؤ¶رُ»¯¹è£¬ت¹ئن×ھ»¯خھبغµم±ب½دµحµؤ¹èثل¸ئ£¬·´س¦خھCaCO3 CaO£«CO2،ü£¬CaO£«SiO2
CaO£«CO2،ü£¬CaO£«SiO2 CaSiO3،£¹èثل¸ئµؤأـ¶ب±ببغبعجْµؤأـ¶بµح£¬ثùزششع¸كآ¯ضذ¹èثل¸ئµبآ¯شüتا¸،شعجْث®ض®ةدµؤ،£
CaSiO3،£¹èثل¸ئµؤأـ¶ب±ببغبعجْµؤأـ¶بµح£¬ثùزششع¸كآ¯ضذ¹èثل¸ئµبآ¯شüتا¸،شعجْث®ض®ةدµؤ،£
(2)½¹ج؟شع¸كآ¯ء¶جْضذµؤ×÷سأتاجل¹©ؤـء؟(×÷خھب¼ءد)،¢×÷خھ»¹ش¼ء،¢»¹سذ¶شئنضذµؤخïءدئًµ½ض§³إ؛حتèة¢µؤ×÷سأ،£
(3)¸كآ¯ء¶جْ²ْةْµؤخ²ئّضذ؛¬سذ´َء؟µؤCO،¢CO2،¢·غ³¾؛حءٍ،¢µھµؤرُ»¯خثùزش¸كآ¯ء¶جْ»ل¼س¾çثلسêµؤ·¢ةْ£¬»¹»لµ¼ضآ¾ض²؟µطاّضذµؤCOإ¨¶ب¹´َ£¬ت¹ضـخ§ةْخïتـµ½¶¾؛¦،£
(4)¸كآ¯ء¶جْضذ£¬¸ù¾ف½¹ج؟µؤ×÷سأ£¬؟ةزشذ´³ِئنب¼ةص·´س¦،¢سë¶رُ»¯ج¼µؤ·´س¦،¢ز»رُ»¯ج¼»¹شرُ»¯جْµؤ·´س¦،£
(5)´س،°ب·د،±ہûسأ،¢»·¾³±£»¤µب½ا¶ب؟¼آا£¬¸ضجْئَزµشعةْ²ْضذس¦½¨ء¢خغث®´¦ہيدµح³£»½«ت¯»زت¯ىرةص³ةةْت¯»زسأسعخüتص·¢µç³§؛ح½¹»¯³§ب¼أ؛ت±²ْةْµؤSO2£¬¼ُةظ¶ش؟صئّµؤخغب¾£»سأ·¢µç³§µؤأ؛ي·ت¯؛ح·غأ؛»ز×÷خھةْ²ْث®ؤàµؤشءد£»سأء¶جْ³§µؤآ¯شü×÷خھةْ²ْث®ؤàµؤشءد،£

 ر§ء·؟ى³µµہ؟ىہض¼ظئعتî¼ظ×÷زµذآ½®بثأٌ³ِ°وةçدµءذ´ً°¸
ر§ء·؟ى³µµہ؟ىہض¼ظئعتî¼ظ×÷زµذآ½®بثأٌ³ِ°وةçدµءذ´ً°¸ صم´َسإر§ذ،ر§ؤ꼶دخ½سµ¼سëء·صم½´َر§³ِ°وةçدµءذ´ً°¸
صم´َسإر§ذ،ر§ؤ꼶دخ½سµ¼سëء·صم½´َر§³ِ°وةçدµءذ´ً°¸ ذ،ر§تî¼ظ×÷زµ¶«ؤد´َر§³ِ°وةçدµءذ´ً°¸
ذ،ر§تî¼ظ×÷زµ¶«ؤد´َر§³ِ°وةçدµءذ´ً°¸ ½ٍاإ½جستî¼ظ°خ¸كدخ½س¹م¶«بثأٌ³ِ°وةçدµءذ´ً°¸
½ٍاإ½جستî¼ظ°خ¸كدخ½س¹م¶«بثأٌ³ِ°وةçدµءذ´ً°¸؛£ث®ضذأ¾µؤ×ـ´¢ء؟ش¼خھ2.1،ء1015t£¬ؤ؟ا°تہ½çةد60%µؤأ¾ہ´×ش؛£ث®£¬¹¤زµ¹وؤ£؛£ث®جلأ¾µؤء÷³جبçح¼ثùت¾£؛
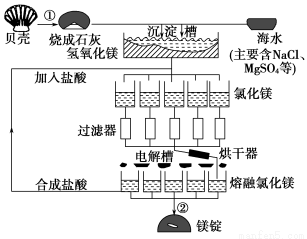
تش»ط´ًدآءذختجâ£؛
(1)؛£ث®جلأ¾µؤ¹³جضذ¢ظ،¢¢ع·´س¦µؤ»¯ر§·½³جت½£؛
¢ظ_____________________________________£¬
¢ع______________________________________،£
(2)سةآب»¯أ¾بـز؛ضئب،خقث®آب»¯أ¾¹ججه£¬ئن²ظ×÷¹³جتا____________________
(3)خھءثت¹MgSO4حêب«×ھ»¯خھMg(OH)2£¬شٍ¼سبëµؤةْت¯»ززھ¹ء؟£¬ب»؛َ·ضہëµأMg(OH)2³ءµي£¬؟¼آاCa(OH)2بـ½â¶ب£¬س¦¸أسأ________·¨·ضہë،£
(4)½ًتôأ¾،¢آءµؤ¹¤زµز±ء¶·½·¨¼بسذدàثئض®´¦£¬سضسذ²»ح¬ض®±ً£¬دآ±يتارُ»¯أ¾؛حآب»¯أ¾µؤبغ·ذµمت¾ف£؛
خïضت | رُ»¯أ¾ | آب»¯أ¾ |
بغµم(،و) | 2 852 | 714 |
·ذµم(،و) | 3 600 | 1 412 |
¹¤زµةدء¶أ¾²ةسأµç½âبغبعآب»¯أ¾£¬¶ّز±ء¶آءشٍسأµç½âبغبعµؤAl2O3£¬ئنشزٍتا____________________________________