��Ŀ����
����Ŀ����֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��
����AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
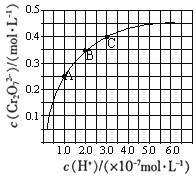
��1����ʯ������A���ѽⷴӦ����_______________��������ѧ���������������仯��
��2��A�Ľṹ��ʽΪ___________��A��һ�������¿��Ծۺ�����һ�ֳ������ϣ������ϵĽṹ��ʽΪ___________��
��3���ٵķ�Ӧ����Ϊ__________��D�й����ŵ�������__________��
��4����ʵ�����л�õ�����������������B��D��Ϊ�ᴿ����������������Լ���_________���������������__________��
��5����Ӧ�ڵĻ�ѧ����ʽΪ__________________________________����Ӧ�ܵĻ�ѧ����ʽΪ_____________________________________��
���𰸡���ѧ CH2=CH2 ![]() �ӳɷ�Ӧ �Ȼ� ����̼������Һ ��Һ 2CH3CH2OH+O2
�ӳɷ�Ӧ �Ȼ� ����̼������Һ ��Һ 2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O CH3CH2OH + CH3COOH
2CH3CHO+2H2O CH3CH2OH + CH3COOH![]() CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O
��������
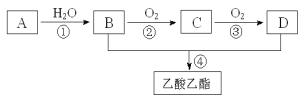
��ϩ�IJ���ͨ����������һ������ʯ�ͻ���ˮƽ����A����ϩ��һ�������£���ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����BΪ�Ҵ����ڴ��������£��Ҵ�������������Ӧ������ȩ����CΪ��ȩ���ڴ��������£���ȩ������������Ӧ�������ᣬ��DΪ�����Ũ���������������£��������Ҵ����ȷ���������Ӧ����������������EΪ����������
��1����ʯ��������ϩ���ѽⷴӦ�������������ɣ����ڻ�ѧ�仯���ʴ�Ϊ����ѧ��
��2��A����ϩ���ṹ��ʽΪCH2=CH2��һ����������ϩ�����Ӿ۷�Ӧ���ɾ���ϩ������ϩ�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��CH2=CH2��
���ʴ�Ϊ��CH2=CH2��![]() ��
��
��3����Ӧ��Ϊһ�������£���ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ���DΪ���ᣬ�ṹ��ʽΪCH3COOH��������Ϊ�Ȼ����ʴ�Ϊ���ӳɷ�Ӧ���Ȼ���
��4��ʵ�����Ƶõ����������л��лӷ�����������Ҵ��������������Ҵ������������м��뱥��̼������Һ�����Գ�ȥ���ᣬ�����Ҵ������������������ܽ�ȱ��ڷֲ㣬��Һ�ռ��õ������������ʴ�Ϊ������̼������Һ����Һ��
��5����Ӧ��Ϊ�ڴ��������£��Ҵ�������������Ӧ������ȩ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O����Ӧ��Ϊ��Ũ���������������£��������Ҵ����ȷ���������Ӧ����������������Ӧ�Ļ�ѧ����ʽΪCH3CH2OH + CH3COOH
2CH3CHO+2H2O����Ӧ��Ϊ��Ũ���������������£��������Ҵ����ȷ���������Ӧ����������������Ӧ�Ļ�ѧ����ʽΪCH3CH2OH + CH3COOH![]() CH3COOCH2CH3 + H2O���ʴ�Ϊ��2CH3CH2OH+O2
CH3COOCH2CH3 + H2O���ʴ�Ϊ��2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O��CH3CH2OH + CH3COOH
2CH3CHO+2H2O��CH3CH2OH + CH3COOH![]() CH3COOCH2CH3 + H2O��
CH3COOCH2CH3 + H2O��

����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ�~����Ԫ�أ���д���пհ�
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
(��Ԫ�ط��Ż�ѧʽ�ش���������)
(1)����ЩԪ���У���ѧ��������õ���_______����ԭ�ӽṹʾ��ͼ��_________��
(2)�õ���ʽ��ʾԪ�آ������ɵĻ�������γɹ��̣�________���û���������_______(��������������������)�����
(3)�����³�Һ̬�ķǽ���������_______��
(4)�������γ��������������Ԫ����_________����Ԫ�صĵ�����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ��___________��
(5)�١��ޡ�������Ԫ�ص�����������Ӧ��ˮ�����У���������ǿ��˳������Ϊ_________��