��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ�~����Ԫ�أ���д���пհ�
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
(��Ԫ�ط��Ż�ѧʽ�ش���������)
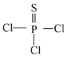
(1)����ЩԪ���У���ѧ��������õ���_______����ԭ�ӽṹʾ��ͼ��_________��
(2)�õ���ʽ��ʾԪ�آ������ɵĻ�������γɹ��̣�________���û���������_______(��������������������)�����
(3)�����³�Һ̬�ķǽ���������_______��
(4)�������γ��������������Ԫ����_________����Ԫ�صĵ�����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ��___________��
(5)�١��ޡ�������Ԫ�ص�����������Ӧ��ˮ�����У���������ǿ��˳������Ϊ_________��
���𰸡�Ar 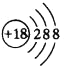
![]() ���� Br2 Al 2Al+2KOH+2H2O=2KAlO2+3H2�� H2CO3��H2SO4��HClO4
���� Br2 Al 2Al+2KOH+2H2O=2KAlO2+3H2�� H2CO3��H2SO4��HClO4
��������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪF����ΪNa����ΪAl����ΪS����ΪCl����ΪAr����ΪK����ΪBr�����ԭ�ӽṹ��Ԫ�������ɷ������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪF����ΪNa����ΪAl����ΪS����ΪCl����ΪAr����ΪK����ΪBr��
(1)����Ԫ���У�����õ��Ƕ���Ԫ�أ�ΪAr����ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��Ar��
���ʴ�Ϊ��Ar�� ��
��
(2)����ߵĻ�����Ϊ�Ȼ��ƣ�Ϊ���ӻ�������γɹ����õ���ʽ��ʾΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() �����ӣ�
�����ӣ�
(3)�����³�Һ̬�ķǽ�������ΪBr2���ʴ�Ϊ��Br2��
(4)����������������������������������ط�Ӧ����ƫ����أ���Ӧ�Ļ�ѧ����ʽΪ2Al+2KOH+2H2O=2KAlO2+3H2�����ʴ�Ϊ��Al��2Al+2KOH+2H2O=2KAlO2+3H2����
(5)Ԫ�صķǽ�����Խǿ������������Ӧ��ˮ���������Խǿ���١��ޡ�������Ԫ�ص�����������Ӧ��ˮ�����У���������ǿ��˳������ΪH2CO3��H2SO4��HClO4���ʴ�Ϊ��H2CO3��H2SO4��HClO4��