��Ŀ����
����Ŀ��Ԫ��C�����Ԫ��Co��Fe��Ni���ڹ�ҵ��ũҵ����ѧ�����Լ����������л��ϳɵȷ�������Ҫ���á�
��1����̬Coԭ�Ӽ۵��ӹ���Ų�ͼΪ______�����ĵ�����I4(Co)��I4(Fe)����ԭ����______��
��2��(CH3)3C+���л��ϳ���Ҫ�м��壬���м�����̼ԭ���ӻ���ʽΪ______��
��3��Co2+��ˮ��Һ����[Co(H2O)6]2+���ڡ���Co2+����Һ�м��������ˮ�����ɸ��ȶ���[Co(NH3)6]2+����ԭ����______��
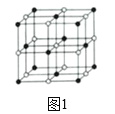
��4��Co��һ��������ľ�����ͼ1����֪��ԭ�ӵİ뾶Ϊapm����ԭ�ӵİ뾶Ϊbpm�������ھ������ǽ��ܽӴ��ģ��ڸ��ܵ������ᄃ����ԭ�ӵĿռ�������Ϊ______���г���a��b�ļ������ʽ���ɣ�����ʾ���������㣬���ж���ԭ��֮���Ƿ���ܽӴ�����
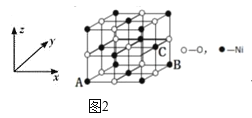
��5��NiO�ľ���ṹ��ͼ2��ʾ�����������������AΪ��0��0��0����BΪ��1��1��0������C�����������Ϊ______��
���𰸡�![]() ��ʧȥ���ǽ��ȶ���3d5��һ�����ӣ���ʧȥ����3d6�ϵ�һ������ sp2��sp3�ӻ� NԪ�ص縺�Ա�OԪ�ص縺��С��Nԭ���ṩ�µ��ӶԵ����������Co2+�γɵ���λ����ǿ
��ʧȥ���ǽ��ȶ���3d5��һ�����ӣ���ʧȥ����3d6�ϵ�һ������ sp2��sp3�ӻ� NԪ�ص縺�Ա�OԪ�ص縺��С��Nԭ���ṩ�µ��ӶԵ����������Co2+�γɵ���λ����ǿ 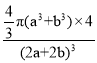 ��100% ��1��
��100% ��1��![]() ��
��![]() ��
��
��������
��1��CoΪ27��Ԫ�أ���������Ų�ʽΪ��1s22s22p63s23p63d74s2�����ڹ���Ԫ�أ��۵��Ӱ���3d��4s���ӣ��۵����Ų�ʽΪ3d74s2���������ԭ�������ع��������ʽΪ![]() ���ɺ��ع�����������ʧȥ����3d6�ϵ�һ�����ӣ�����ʧȥ���ǽ��ȶ���3d5��һ�����ӣ��ʵ��ĵ�����I4��Co����I4��Fe����
���ɺ��ع�����������ʧȥ����3d6�ϵ�һ�����ӣ�����ʧȥ���ǽ��ȶ���3d5��һ�����ӣ��ʵ��ĵ�����I4��Co����I4��Fe����
�ʴ�Ϊ��![]() �� ��ʧȥ����3d6�ϵ�һ�����ӣ�����ʧȥ���ǽ��ȶ���3d5��һ�����ӣ�
�� ��ʧȥ����3d6�ϵ�һ�����ӣ�����ʧȥ���ǽ��ȶ���3d5��һ�����ӣ�
��2��(CH3)3C+���л��ϳ���Ҫ�м��壬����̼ԭ���γ�4������������̼ԭ���γ�3����������û�йµ��Ӷԣ��ӻ������Ŀ�ֱ�Ϊ4��3���ֱ��ȡsp3��sp2�ӻ���
�ʴ�Ϊ��sp2��sp3�ӻ�����
��3��Co2+��ˮ��Һ����[Co(H2O)6]2+���ڡ���Co2+����Һ�м��������ˮ�����ɸ��ȶ���[Co(NH3)6]2+����ԭ���ǣ�NԪ�ص縺�Ա�OԪ�ص縺��С��Nԭ���ṩ�µ��ӶԵ����������Co2+�γɵ���λ����ǿ��
�ʴ�Ϊ�� NԪ�ص縺�Ա�OԪ�ص縺��С��Nԭ���ṩ�µ��ӶԵ����������Co2+�γɵ���λ����ǿ��
��4���ɾ����ṹ��֪��������Co��Oԭ�ӵ���λ����Ϊ6����ԭ��������ԭ�ӵ����������϶�У���ԭ�������Coԭ�ӵ����������϶�С�������Coԭ����Ŀ=1+12��![]() =4��Oԭ����Ŀ=8��
=4��Oԭ����Ŀ=8��![]() +6��
+6��![]() =4��������ԭ�������=4��
=4��������ԭ�������=4��![]() �������ⳤΪ��2a+2b��pm���������=��2a+2b��3���ռ�������=
�������ⳤΪ��2a+2b��pm���������=��2a+2b��3���ռ�������=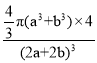 ��100% ���ʴ�Ϊ��
��100% ���ʴ�Ϊ��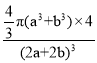 ��100% ��
��100% ��
��5������NiO�ľ���ṹ�����������������AΪ��0��0��0����BΪ��1��1��0������C�����������Ϊ��1��![]() ��
��![]() ����
����
�ʴ�Ϊ����1��![]() ��
��![]() ����
����

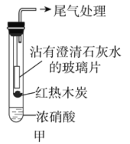
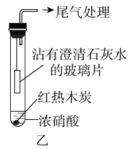
����Ŀ������ʵ�������װ���ܴﵽĿ�ĵ��ǣ� ��
A | B | C | D |
|
|
|
|
���Ũ������Ҵ� | ����һ��Ũ�ȵ���Һ | �ռ� | ֤����Ȳ��ʹ��ˮ��ɫ |
A. AB. BC. CD. D
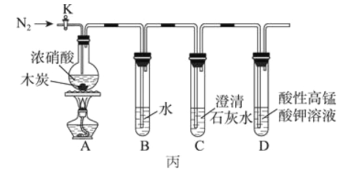
����Ŀ��ϩ������Ҫ���л�����ԭ�ϡ�ʵ������Ҫ��Ũ�����Ũ����������ʹ����ˮ��ȡϩ����ijͬѧʹ�û�������ˮ�Ʊ�����ϩ����Ʒ������£�
��һ����Ҫ�������Լ�
������50mLԲ����ƿ����������ֱ�������ܡ�10mL��Ͳ����Һ©����100mL��ƿ������ͷ����Һ�ܡ�
�Լ���10.0g(10.4mL��0.1mol)��������5mLŨ���ᡢ�Ȼ��ơ���ˮ�Ȼ��ơ�5%̼����ˮ��Һ��
����������ʵ�����漰�ķ�Ӧ�����������ĸ����������ʣ��б����£�
��ѧ���� | ��Է��� ���� | ����ܶ�/ g��cm-3 | �е�/�� | �ܽ��� |
������ | 100 | 0.96 | 161.1 | ������ˮ |
����(85%) | 98 | 1.83 | 213 | ������ˮ |
����ϩ | 82 | 0.89 | 83.3 | ����ˮ |
������ʵ������

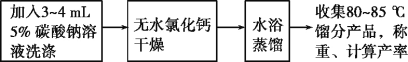
��ش�
��1�����ȹ����У������Ǽӷ�ʯ��Ӧ��β���?___
��2�����ֲ�Ʒ��ȥˮ������Ҫ�õ�����Ҫʵ��������___��
��3����ʵ����Ũ�������Ũ������ŵ㣺___��
��4����ʵ�����Ҫ������Ϊ___(����������)��
��5�����ᴿ����ϩʱ���õ�����ı���ʳ��ˮ��������ˮ��ԭ����___������3��4mL5%̼������Һ��Ŀ����___��
��6��ˮԡ�������õ�7.0g��Ʒ����Ӧ�IJ���Ϊ___(����2λ��Ч����)��