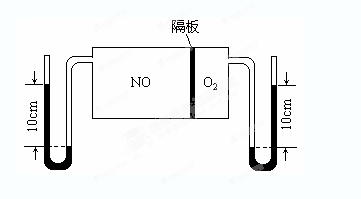
��Ŀ����
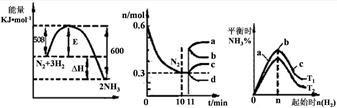
��ͬ�¶��£������Ϊ0. 25 L�����������ܱ������з������淴Ӧ��N2(g)��3H2(g) 2NH3(g)����H����92.6 kJ/mol��ʵ������ʼʱ���й����������ʾ��
2NH3(g)����H����92.6 kJ/mol��ʵ������ʼʱ���й����������ʾ��
���������������(����)
A�������٢��з�Ӧ��ƽ�ⳣ�����
B��ƽ��ʱ������������NH3�����������Ϊ
C���������дﵽƽ��ʱ�ų�������Q��23.15 kJ
D�������������Ϊ0.5 L����ƽ��ʱ�ų�������С��23.15 kJ
 2NH3(g)����H����92.6 kJ/mol��ʵ������ʼʱ���й����������ʾ��
2NH3(g)����H����92.6 kJ/mol��ʵ������ʼʱ���й����������ʾ��| ���� ��� | ��ʼʱ�����ʵ����ʵ���/mol | �ﵽƽ��ʱ�� ϵ�����ı仯 | ||
| N2 | H2 | NH3 | ||
| �� | 1 | 3 | 0 | �ų�������23.15 kJ |
| �� | 0.9 | 2.7 | 0.2 | �ų�������Q |
���������������(����)
A�������٢��з�Ӧ��ƽ�ⳣ�����
B��ƽ��ʱ������������NH3�����������Ϊ

C���������дﵽƽ��ʱ�ų�������Q��23.15 kJ
D�������������Ϊ0.5 L����ƽ��ʱ�ų�������С��23.15 kJ
C
���ڸ�����Ӧ��ƽ�ⳣ��ֻ���¶ȵĺ������¶���ͬ��ƽ�ⳣ����ͬ��A��ȷ���ɢ��зų�����������֪�μӷ�Ӧ��N2Ϊ0.25 mol������
������ N2(g)��3H2(g) 2NH3(g)
2NH3(g)
n(ʼ)/mol��1������ 3�������� 0
n(��)/mol 0.25 0.75 0.5
n(ƽ)/mol 0.75 2.25 0.5
�����NH3���������Ϊ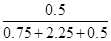 ��
�� �����ڢٺ͢��н�����ƽ������ͬ�ģ�������������NH3�����������Ϊ
�����ڢٺ͢��н�����ƽ������ͬ�ģ�������������NH3�����������Ϊ ��B��ȷ���ٺ͢ڽ���������ͬ��ƽ�⣬����N2ת��0.15 mol���ų�������Ϊ92.6 kJ/mol��0.15 mol��13.89 kJ��C����ȷ�������������Ϊ0.5 L������Ӧ�������ѹǿ��С��ƽ�����淴Ӧ�����ƶ�����ƽ��ʱ�ų�������С��23.15 kJ��D��ȷ��
��B��ȷ���ٺ͢ڽ���������ͬ��ƽ�⣬����N2ת��0.15 mol���ų�������Ϊ92.6 kJ/mol��0.15 mol��13.89 kJ��C����ȷ�������������Ϊ0.5 L������Ӧ�������ѹǿ��С��ƽ�����淴Ӧ�����ƶ�����ƽ��ʱ�ų�������С��23.15 kJ��D��ȷ��
������ N2(g)��3H2(g)
 2NH3(g)
2NH3(g)n(ʼ)/mol��1������ 3�������� 0
n(��)/mol 0.25 0.75 0.5
n(ƽ)/mol 0.75 2.25 0.5
�����NH3���������Ϊ
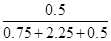 ��
�� �����ڢٺ͢��н�����ƽ������ͬ�ģ�������������NH3�����������Ϊ
�����ڢٺ͢��н�����ƽ������ͬ�ģ�������������NH3�����������Ϊ ��B��ȷ���ٺ͢ڽ���������ͬ��ƽ�⣬����N2ת��0.15 mol���ų�������Ϊ92.6 kJ/mol��0.15 mol��13.89 kJ��C����ȷ�������������Ϊ0.5 L������Ӧ�������ѹǿ��С��ƽ�����淴Ӧ�����ƶ�����ƽ��ʱ�ų�������С��23.15 kJ��D��ȷ��
��B��ȷ���ٺ͢ڽ���������ͬ��ƽ�⣬����N2ת��0.15 mol���ų�������Ϊ92.6 kJ/mol��0.15 mol��13.89 kJ��C����ȷ�������������Ϊ0.5 L������Ӧ�������ѹǿ��С��ƽ�����淴Ӧ�����ƶ�����ƽ��ʱ�ų�������С��23.15 kJ��D��ȷ��
��ϰ��ϵ�д�
�����Ŀ
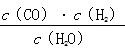 ����ش��������⡣
����ش��������⡣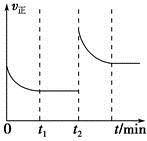
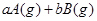

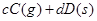
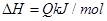 ����Ӧ�����У���������������ʱ��C�ڻ�����еĺ������¶ȣ�T���Ĺ�ϵ��ͼI��ʾ����Ӧ���ʣ�v����ѹǿ��p���Ĺ�ϵ��ͼII��ʾ����ͼ����������˵����ȷ���ǣ�������
����Ӧ�����У���������������ʱ��C�ڻ�����еĺ������¶ȣ�T���Ĺ�ϵ��ͼI��ʾ����Ӧ���ʣ�v����ѹǿ��p���Ĺ�ϵ��ͼII��ʾ����ͼ����������˵����ȷ���ǣ�������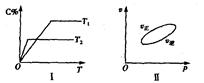
 2SO3(g)��ƽ������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ��������ͼ�ش��������⣺
2SO3(g)��ƽ������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ��������ͼ�ش��������⣺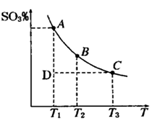
 CH3OH(g)��H2O(g)��5 min��Ӧ�ﵽƽ��ʱc(CH3OH)Ϊ0.2 mol��L��1��CO2(g)��ƽ�����ʵ���Ũ��c(CO2)���¶ȹ�ϵ��ͼ��ʾ������˵���������(����)��
CH3OH(g)��H2O(g)��5 min��Ӧ�ﵽƽ��ʱc(CH3OH)Ϊ0.2 mol��L��1��CO2(g)��ƽ�����ʵ���Ũ��c(CO2)���¶ȹ�ϵ��ͼ��ʾ������˵���������(����)��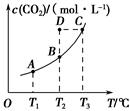
 4NO2(g)��O2(g) ���SH��0
4NO2(g)��O2(g) ���SH��0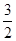 ��(������NO2�ۺϳ�N2O4)����N2O5��ת����a1�� �����¶��·�Ӧ��ƽ�ⳣ��K��_______��
��(������NO2�ۺϳ�N2O4)����N2O5��ת����a1�� �����¶��·�Ӧ��ƽ�ⳣ��K��_______��