��Ŀ����
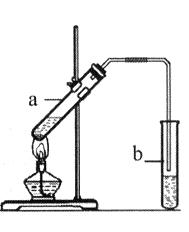
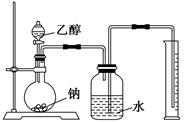
�Ҵ���һ�������¿ɱ�������ijͬѧ��������װ�ý����Ҵ��Ĵ�����ʵ�飬��������������Cװ�õ��Թ���ʢ����ˮ�Ҵ�����֪��ȩ�ڼ�����������������Cu��OH��2����Һ��Ӧ����Cu2O���������ƾ��ƣ�
�ش��������⣺
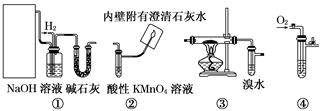
��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ __���������ӿڵ���ĸ��ţ������Ⱥͼг�װ������ȥ����
��2��Bװ�õķ�Һ©����Һ��ҩƷ�� _���ѧʽ������Һ�廹����������KMnO4��Һ��Ӧ������ͬ�����壬д���÷�Ӧ�����ӷ���ʽ ��
��3��Fװ���еĹ����Լ��� ��������Ӧ�Ļ�ѧ����ʽΪ ����Ӧһ��ʱ�����ȥ�ƾ��ƣ���Ӧ�ܼ������У���ԭ���� ��
��4��Aװ�ú�Dװ���е�ʵ������ֱ�Ϊ �� ��
��5��Ϊ������Ҵ���ת���ʣ����Զ�Cװ�ý��иĽ��������ĸĽ���� ��
��1��c��hi��de��jk����kj����fg����gf����a
��2��H2O2��2MnO4-��5H2O2��6H��=2Mn2����8H2O��5O2��
��3��ͭ˿��2CH3CH2OH��O2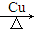 2CH3CHO��2H2O���÷�ӦΪ���ȷ�Ӧ����Ӧ���̷ų���������ά�ַ�Ӧ��������
2CH3CHO��2H2O���÷�ӦΪ���ȷ�Ӧ����Ӧ���̷ų���������ά�ַ�Ӧ��������
��4��A�г���ש��ɫ������D�а�ɫ��ĩ��Ϊ��ɫ
��5��ʹ���¶ȼƣ�����Cװ����ˮԡ���¶��Ը����Ҵ��е㣨�����������𰸣�
����

���ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ���ڵ�ͬ���칹�����Ʋ��Ҵ��Ľṹ��������������֮һ��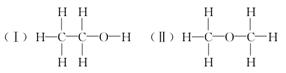
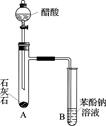
Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�顣�ָ����Ҵ����ơ�ˮ����Ҫ����������ס��ҡ�����������ͬѧֱ��������ͼ����װ�ý���ʵ��ȷ���Ҵ��Ľṹ��
(1)ѧ���õ�һ��ʵ�����ݣ�
| �Ҵ������ʵ���(mol) | ���������(L) |
| 0.10 | 1.12(��״��) |
�������������ƶ��Ҵ��ĽṹӦΪ________(�â��ʾ)������Ϊ_______________��
(2)ͬѧ�ҷֱ�ȷ����4.60 g�Ҵ����ж��ʵ�飬����������ŵ���Ͳ�ڵ�ˮ�������Ϊ���ɵ�H2�������ɱ�״����С��1.12 L�����������Ͳ�������Ҷ�����ɵ�����ô����Ϊ������������Ʒ�к�������ˮ��ɵģ�����Ϊ��ȷ��________(���ȷ������ȷ��)�������Ϊ��ȷ����˵�����ɣ��������Ϊ����ȷ���Dz������������ԭ��Ӧ����ʲô��________________��
(3)ͬѧ����Ϊʵ��ɹ��Ĺؼ��У���װ��������Ҫ����
��ʵ�鿪ʼǰȷȷ���Ҵ������������������ܹ��ƿ��ˮ�������������������IJ��㷽����ȷ������ȷ��������ȷ����________��(�����)
(4)ͬѧ������ͨ�������Ҵ���������ȷ���Ҵ���������ô������֪����������_____________��
(5)ʵ�������ͬѧ���Ҵ��Ŀ��ܽṹ�������ֶ��Ҵ����Ƶ����Ĺ�ϵ���������ۣ�����Ҵ������ʵ���Ϊn mol����ô���Ƶ����ʵ�����ȡֵҪ�������_________________��
ij�л���Ľṹ��ʽΪCH3CCl=CHCHO,���и����У����л��ﲻ���ܷ����Ļ�ѧ��Ӧ�� ��
��������Ӧ ��ȡ����Ӧ �ۼӳɷ�Ӧ ����ȥ��Ӧ �ݻ�ԭ��Ӧ ��������Ӧ ��ˮ�ⷴӦ ��ۺϷ�Ӧ
| A���� | B���� | C���� | D���� |
���и����еķ�Ӧ����ͬһ��Ӧ���͵���( )
| A������������Ӧ����ϩʹ��ˮ��ɫ |
| B��������������������ͭ���ȣ���ˮ�Ҵ���Ũ���Ṳ������ϩ |
| C���Ҵ�����������������������������õ������� |
| D���Ҵ�����������ȩ������Һ�����屽 |
 2________+2C2H5OH
2________+2C2H5OH