��Ŀ����
����Ŀ�������������ʵ���Ҫ���������ʵĽṹ����ش��������⡣
(1)��֪X��YΪ��������Ԫ��,��ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
������/(kJ/mol) | I1 | I2 | I3 | I4 |
X | 578 | 1817 | 2745 | 11578 |
Y | 738 | 1451 | 7733 | 10540 |
Xͨ����_____�ۣ�X�ĵ縺��____Y�ĵ縺��(�>���� =����<��)��
(2)�����Ĺ��������е�����ԼΪ399kJ/mol�������±��йص����ʷ�������Ҫ��ѧ������Ϣ��˵�����峤ʱ������������Ƥ�������˺���ԭ��_______________��
���ۼ� | C-C�� | C-N�� | C-S�� |
����/(kJ/mol) | 347 | 305 | 259 |
��ɵ����ʵ���İ������е�̼ԭ�ӵ��ӻ�������__________��
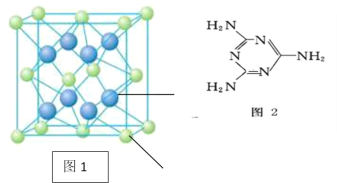
(3)ʵ��֤��:KCl��MgO��CaO��TiN�����־���Ľṹ��NaCl����ṹ����(��ͼ),����3�����Ӿ���ľ������������±�:
���Ӿ��� | NaCl | KCl | CaO |
������/(kJ.mol-1) | 786 | 715 | 3401 |

�����������Ӿ�����۵�Ӹߵ��͵�˳����_______������MgO������һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+��______����
(4)���������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á������ͻ�����V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���_________��
���𰸡� +3 > �������е������ȵ����ʷ����е���Ҫ��ѧ��C-C����C-N����C-S���ļ��ܶ�����������������ʹ��Щ��ѧ�����ѣ��Ӷ��ƻ������ʷ��� sp2��sp3 TiN>MgO>CaO>KCl 12 CrO2
�������� (1)�ɱ������ݿ�֪��X�ĵ��ĵ���������������ܵIJ�ֵԶԶ���ڵ�����������ڶ��������Լ��ڶ����������һ�����ܵIJ�ֵ������X���������3�����ӣ�Xͨ����+3����ͬ����Y���������2�����ӣ���ΪX��Y��ͬһ���ڣ�����Y�Ľ����Ա�Xǿ��X�ĵ縺��>Y�ĵ縺�ԡ�
(2)�����Ĺ��������е�����ԼΪ399kJ/mol���ɱ����йص����ʷ�������Ҫ��ѧ������Ϣ�����Է������峤ʱ������������Ƥ�������˺���ԭ���������е������ȵ����ʷ����е���Ҫ��ѧ��C-C����C-N����C-S���ļ��ܶ�����������������ʹ��Щ��ѧ�����ѣ��Ӷ��ƻ������ʷ��ӡ���ɵ����ʵ���İ��������Ұ��������������2��Cԭ�ӣ�һ���γ���̼��˫������һ���DZ��͵ģ�����̼ԭ�ӵ��ӻ�������sp2��sp3��
(3)�ɱ�����Ϣ��֪���ṹ���Ƶ����Ӿ��壬�侧���ܵĴ�С�����Ӱ뾶�����ӵ�����������йأ����Ӱ뾶ԽС�����ӵ��Խ�࣬����Խ�������ۡ��е��Խ�ߡ���������������Ӿ�����۵�Ӹߵ��͵�˳����TiN>MgO>CaO>KCl����MgO����ľ����ṹ��֪��һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+��12����
(4)���������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á������ͻ�����V2O5��CrO2�У�V5+��Cr4+���е�δ�ɶԵ������ֱ�Ϊ0��2�����ԣ��ʺ���¼�����ŷ�ԭ�ϵ���CrO2��