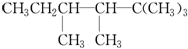��Ŀ����
��1�����������������������ֳ�����������д�ڿհ״���
�������������Ũ�����в��ܽ⣮
�����ձ��г��ڷ���Ũ����ʱ���������ӣ�
���ò�����պŨ�������ֽ��ʱ��ֽ��ڣ�
��2�������Ƶ���ˮ�ֳ����ݣ�
�������е�һ���е���AgNO3��Һ�����ӷ���ʽ�� ��
����ڶ����е���NaHCO3��Һ�����ӷ���ʽ�� ��
����������е�����ɫʯ����Һ���������� ��
��3����ҵ����ȡƯ�۵Ļ�ѧ����ʽ�� ��Ư������ˮ��ˮ�е�CO2��Ӧ�����ɾ���Ư���Ե����ʣ��仯ѧ����ʽ�� ��
�������������Ũ�����в��ܽ⣮
�����ձ��г��ڷ���Ũ����ʱ���������ӣ�
���ò�����պŨ�������ֽ��ʱ��ֽ��ڣ�
��2�������Ƶ���ˮ�ֳ����ݣ�
�������е�һ���е���AgNO3��Һ�����ӷ���ʽ��
����ڶ����е���NaHCO3��Һ�����ӷ���ʽ��
����������е�����ɫʯ����Һ����������
��3����ҵ����ȡƯ�۵Ļ�ѧ����ʽ��
���㣺Ũ���������,�����Ļ�ѧ����
ר�⣺
��������1���ٳ����£�Ũ����������������ۻ�����
��Ũ���������ˮ�ԣ�
��Ũ�����ܽ��л����е�H��OԪ����ˮ������ʽ��ȥ��
��2��������ˮ������Cl2+H2O?HClO+HCl����Һ�к���Cl2��HClO��H2O�ȷ��ӣ�����H+��ClO-��Cl-�����ӣ���϶�Ӧ���ӵ����ʽ����⣻
��3��������ʯ���鷴Ӧ�Ʊ�Ư�ۣ�Ư������ˮ��ˮ�е�CO2��Ӧ�����ɾ���Ư���Ե�����ΪHClO���Դ������
��Ũ���������ˮ�ԣ�
��Ũ�����ܽ��л����е�H��OԪ����ˮ������ʽ��ȥ��
��2��������ˮ������Cl2+H2O?HClO+HCl����Һ�к���Cl2��HClO��H2O�ȷ��ӣ�����H+��ClO-��Cl-�����ӣ���϶�Ӧ���ӵ����ʽ����⣻
��3��������ʯ���鷴Ӧ�Ʊ�Ư�ۣ�Ư������ˮ��ˮ�е�CO2��Ӧ�����ɾ���Ư���Ե�����ΪHClO���Դ������
���
�⣺��1���ٳ����£�Ũ����������������ۻ������������������Ũ�����в��ܽⷢ���ۻ����ʴ�Ϊ���ۻ���
��Ũ���������ˮ�ԣ������ձ��г��ڷ���Ũ����ʱ���������ӣ�����ˮ���йأ��ʴ�Ϊ����ˮ�ԣ�
��Ũ�����ܽ��л����е�H��OԪ����ˮ������ʽ��ȥ�����ò�����պŨ�������ֽ��ʱ��ֽ��ڣ�����ˮ���йأ��ʴ�Ϊ����ˮ�ԣ�
��2��������ˮ������Cl2+H2O?HClO+HCl����Һ�к���Cl2��HClO��H2O�ȷ��ӣ�����H+��ClO-��Cl-�����ӣ���
�������е�һ���е���AgNO3��Һ���������ӷ�Ӧ���ɳ��������ӷ���ʽ��Cl-+Ag+=AgCl�����ʴ�Ϊ��Cl-+Ag+=AgCl����
����ڶ����е���NaHCO3��Һ���������ӷ�Ӧ����ˮ�����壬���ӷ���ʽ��H++HCO3-=CO2��+H2O���ʴ�Ϊ��H++HCO3-=CO2��+H2O��
�ۺ������Ӻ�HClO����������е�����ɫʯ����Һ���������ȱ�����ɫ���ʴ�Ϊ��������ɫ��
��3��Ư�۵���Ҫ�ɷ��Ǵ������[Ca��ClO��2]���Ȼ��ƣ���ҵ�Ͻ�����ͨ��ʯ����[Ca��OH��2]��ȡƯ�ۣ�ͬʱ��ˮ���ɣ���Ӧ�Ļ�ѧ����ʽΪ��2Cl2+2Ca��OH��2=Ca��ClO��2+CaCl2+2H2O��
Ư������ˮ����CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬͬʱ����̼��Ƴ�������Ӧ�Ļ�ѧ����ʽΪ��Ca��ClO��2+CO2+H2O=CaCO3��+2HClO��
�ʴ�Ϊ��2Cl2+2Ca��OH��2=Ca��ClO��2+CaCl2+2H2O��Ca��ClO��2+CO2+H2O=CaCO3��+2HClO��
��Ũ���������ˮ�ԣ������ձ��г��ڷ���Ũ����ʱ���������ӣ�����ˮ���йأ��ʴ�Ϊ����ˮ�ԣ�
��Ũ�����ܽ��л����е�H��OԪ����ˮ������ʽ��ȥ�����ò�����պŨ�������ֽ��ʱ��ֽ��ڣ�����ˮ���йأ��ʴ�Ϊ����ˮ�ԣ�
��2��������ˮ������Cl2+H2O?HClO+HCl����Һ�к���Cl2��HClO��H2O�ȷ��ӣ�����H+��ClO-��Cl-�����ӣ���
�������е�һ���е���AgNO3��Һ���������ӷ�Ӧ���ɳ��������ӷ���ʽ��Cl-+Ag+=AgCl�����ʴ�Ϊ��Cl-+Ag+=AgCl����
����ڶ����е���NaHCO3��Һ���������ӷ�Ӧ����ˮ�����壬���ӷ���ʽ��H++HCO3-=CO2��+H2O���ʴ�Ϊ��H++HCO3-=CO2��+H2O��
�ۺ������Ӻ�HClO����������е�����ɫʯ����Һ���������ȱ�����ɫ���ʴ�Ϊ��������ɫ��
��3��Ư�۵���Ҫ�ɷ��Ǵ������[Ca��ClO��2]���Ȼ��ƣ���ҵ�Ͻ�����ͨ��ʯ����[Ca��OH��2]��ȡƯ�ۣ�ͬʱ��ˮ���ɣ���Ӧ�Ļ�ѧ����ʽΪ��2Cl2+2Ca��OH��2=Ca��ClO��2+CaCl2+2H2O��
Ư������ˮ����CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬͬʱ����̼��Ƴ�������Ӧ�Ļ�ѧ����ʽΪ��Ca��ClO��2+CO2+H2O=CaCO3��+2HClO��
�ʴ�Ϊ��2Cl2+2Ca��OH��2=Ca��ClO��2+CaCl2+2H2O��Ca��ClO��2+CO2+H2O=CaCO3��+2HClO��
���������⿼��Ũ��������ʺ����������ʣ�Ϊ��Ƶ���㣬�ۺϿ���Ԫ�ػ�����֪ʶ�����շ����ķ�ӦΪ���Ĺؼ���ע�ػ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
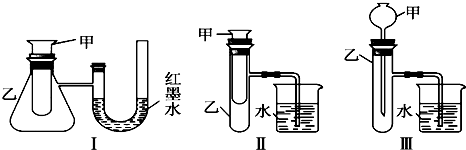
 ��������Ϣ��Ƭ��ͼ��ʾ��
��������Ϣ��Ƭ��ͼ��ʾ��