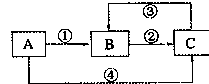
��Ŀ����
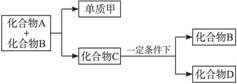
����A������ͼ��ʾת����ϵ��������Ϊ�������ʣ������£�����G ��Ũ��Һ�з����ۻ��� F ����Һ��ֻ����һ�����ʣ��еķ�Ӧ������ˮ��Һ�н��У��еķ�Ӧ��������δȫ����������Ӧ����Ҳδע�������������������������ע����������¸���ĸ���������ʿ��ܲ�ͬ��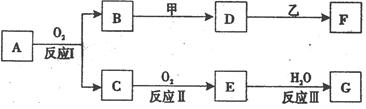
(1)��һ�������AΪ���壻�������������ֱ�պȡ A��G��Ũ��Һ��ʹ���ǽӽ�ʱ���д����������ɣ���Ϊ��ɫ��Ӧ�ʻ�ɫ�Ľ������ʣ� D �� F ����Һ���ʼ��ԡ���
�� B���Ӧ�Ļ�ѧ����ʽΪ____________________ ___________________��
�� D���ҷ�Ӧ�����ӷ���ʽΪ__________________________ _________��
(2)�ڶ��������AΪ��ɫ���壻�������ֵ���ֱ�ӻ��ϵõ��� D ��ˮ��Һ�����������ữ��AgNO3��Һ�а�ɫ�������ɡ���
�� ��ҵ�ϣ���Ӧ I ��___________________�����豸���ƣ��н��С�
�� �Ļ�ѧʽΪ______________��
�� D���ҷ�Ӧ�����ӷ���ʽΪ______________________ _________��
�� ���������G��Һ�������ӵķ���
�� ��A����Է�������ΪM����Ӧ���Ϊ��ȫת������ȡm�˺�A���������ʵ���Ʒ�����������̳�ַ�Ӧ�����ʲ����뷴Ӧ�����õ��ܶ�Ϊ��g/cm3��������������Ϊa% ��G��Һn mL�������Ʒ��A������������ ���г�����ʽ���ɣ���
��1����2Na +2H2O �� 2NaOH + H2�� ��2�֣�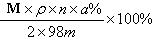
��2Al+2OH��+2H2O �� 2AlO2��+3H2�� ��2�֣�
��2���ٷ���¯ ��1�֣���HCl ��1�֣� ��2Fe3+ + Fe ��3Fe2+ ��2�֣�
��ȡ����G��Һ��һ�Թ��У������еμ�ϡ���ᣬ�ټ���BaCl2��Һ,�а�ɫ�������ɣ�˵����Һ����SO42�� ��2�֣�
��
��2�֣�
����

 ��У����ϵ�д�
��У����ϵ�д�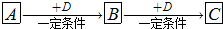
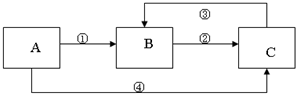
 �� 12�֣�A��B��C���������о�����ͬһ��Ԫ�أ�����֮��������ͼ��ʾ��ת����ϵ�����ַ�Ӧ��������ȥ����
�� 12�֣�A��B��C���������о�����ͬһ��Ԫ�أ�����֮��������ͼ��ʾ��ת����ϵ�����ַ�Ӧ��������ȥ����