��Ŀ����
���͡�������š��ɴ��Ļ��ȼ�ϳ�Һ̬˫��ˮ�⣬������һ��Һ̬���⻯�����֪�û���������Ԫ�ص���������Ϊ12�� 5%����Է�������Ϊ32���ṹ�������ָ÷��ӽṹ��ֻ�е�����
��1���õ��⻯����ĵ���Ϊ ��
��2������������Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
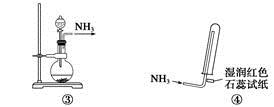
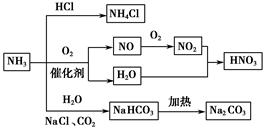
��3��NH3�����е�Nԭ����һ�Թ¶Ե��ӣ��ܷ�����Ӧ��NH3+HCl=NH4Cl����д���������⻯����ͨ����������ʱ��������Ӧ�Ļ�ѧ����ʽ
��1���õ��⻯����ĵ���Ϊ ��
��2������������Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��NH3�����е�Nԭ����һ�Թ¶Ե��ӣ��ܷ�����Ӧ��NH3+HCl=NH4Cl����д���������⻯����ͨ����������ʱ��������Ӧ�Ļ�ѧ����ʽ
��1��N2H4�ĵ���ʽ��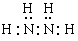 ��2��N2H4+2H2O2=N2+4H2O��3��N2H4+2HCl= N2H6Cl2��
��2��N2H4+2H2O2=N2+4H2O��3��N2H4+2HCl= N2H6Cl2��
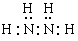 ��2��N2H4+2H2O2=N2+4H2O��3��N2H4+2HCl= N2H6Cl2��
��2��N2H4+2H2O2=N2+4H2O��3��N2H4+2HCl= N2H6Cl2�������������1�����ڸû��������Է�������Ϊ32����Ԫ�ص���������Ϊ12�� 5%������H��32��12�� 5%=4��N��(32-4)��14=2�����Ը����ʵķ���ʽΪN2H4�������ڸ÷��ӽṹ��ֻ�е��������������ʽΪ��
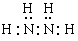 ����2��N2H4��Һ̬˫��ˮ��Ӧ�����������ֲ���Ⱦ��������̬���ʣ��÷�Ӧ�Ļ�ѧ����ʽΪN2H4+2H2O2=N2+4H2O����3���������⽫N2H4ͨ����������ʱ��������Ӧ�Ļ�ѧ����ʽΪN2H4+2HCl= N2H6Cl2��2H4�ķ���ʽ���ƶϡ��ṹ�����ʵ�֪ʶ��
����2��N2H4��Һ̬˫��ˮ��Ӧ�����������ֲ���Ⱦ��������̬���ʣ��÷�Ӧ�Ļ�ѧ����ʽΪN2H4+2H2O2=N2+4H2O����3���������⽫N2H4ͨ����������ʱ��������Ӧ�Ļ�ѧ����ʽΪN2H4+2HCl= N2H6Cl2��2H4�ķ���ʽ���ƶϡ��ṹ�����ʵ�֪ʶ��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ