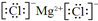��Ŀ����
������Ԫ�����ڱ���һ���֣�����Ԫ���������ڱ��е�λ�ã����û�ѧ����ش��������⣺
�� ���� | IA |
| 0 | |||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A |
|
2 |
|
|
| �� | �� | �� |
|
|
3 | �� |
| �� | �� |
|
| �� |
|
��1��������������ԭ�Ӱ뾶�ɴ�С��˳��Ϊ(Ԫ�ط���)________________________��
��2����������������ۺ������������ǿ������˳����(�ѧʽ)________????? ��
��3�����������������е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��_______________��
��4������������ɵĻ�����������ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ:_______��
��5������������������������ˮ���ﷴӦ�����ӷ���ʽΪ???????????????????? ��
��6�������٢��������л�����Ϊȼ�ϵ�ص�ԭ�ϣ���д���ڼ��Խ�����ȼ�ϵ�ظ����ĵ缫��Ӧʽ:?????????????????????????????????????????????????? ?????? ��
��7��ȼú�����е������������NOx����������̼�����壬�������з�����ȼú����������������ʱ�������ü������ԭ����������
�磺CH4(g)��4NO2(g)=4NO(g)��CO2(g)��2H2O(g) �� ��H =��574 kJ��mol��1
CH4(g)��4NO(g)=2N2(g)��CO2(g)��2H2O(g)? �� ��H =��1160 kJ��mol��1
��CH4(g)��NO2(g)��ԭΪN2(g)�����Ȼ�ѧ����ʽΪ??????????????????????????? ? ��
��1��Na��Al��O������2�֣�
��2��HNO3��H2CO3��H2SiO3 (H4SiO4) ������2�֣�
��3��NaOH��NaClO������2�֣�
��4��2Mg + CO2 2MgO + C������? ��2����
2MgO + C������? ��2����
��5��2Al + 2OH- + 2H2O =2AlO2- + 3H2����������2����
��6��CH4��8e�� ��10OH��=CO32�� ��7H2O��������2����
��7��CH4(g)��2NO2(g)=N2(g)��CO2(g)��2H2O(g)?? ��H=��867 kJ��mol��1 �� ��2����
��������
�������������Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl����
��1��Na��Alλ��ͬһ���ڣ�Naԭ�Ӱ뾶����Al��Oԭ�Ӻ�����Ӳ������٣�ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��Na��Al��O��
��2���ǽ����ԣ�N��C��Si��Ԫ�صķǽ�����Խǿ����Ӧ����ۺ����������Խǿ�������ԣ�HNO3��H2CO3��H2SiO3��
��3�����������������е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ�����ΪNaOH��NaClO��
��4������������ɵĻ����������Ƕ�����̼��һ����̼������ͬ������������Ԫ�صĵ�����þ��þ���������̼��Ӧ��������þ��̼����Ӧ����ѧ����ʽΪ2Mg + CO2 2MgO + C��
2MgO + C��
��5������������������������������ˮ��������������Ӧ�����ӷ���ʽΪ2Al + 2OH- + 2H2O =2AlO2- + 3H2����
��6���٢��������л����Ǽ��飬������Ϊȼ�ϵ���м����ڸ���ͨ�룬�����ڼ��Խ�����ȼ�ϵ�ظ����ĵ缫��ӦʽΪCH4��8e�� ��10OH��=CO32�� ��7H2O��
��7����֪��CH4(g)��4NO2(g)=4NO(g)��CO2(g)��2H2O(g) ��H =��574 kJ��mol��1����CH4(g)��4NO(g)=2N2(g)��CO2(g)��2H2O(g) ��H =��1160 kJ��mol��1������ݸ�˹���ɿ�֪����+�ڣ���2���õ�CH4(g)��NO2(g)��ԭΪN2(g)�����Ȼ�ѧ����ʽ����CH4(g) ��2NO2(g)=N2(g) ��CO2(g) ��2H2O(g)�����Ը÷�Ӧ�ķ�Ӧ����H=����574 kJ��mol��1��1160 kJ��mol��1����2=��867 kJ��mol��1��
���㣺����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ��

��7�֣�Ԫ�����ڱ���ѧϰ���ʽṹ�����ʵ���Ҫ���ߣ�������Ԫ�����ڱ���һ���֣�����������ĸA��D��E��G��Q��M��R��T�ֱ����ijһ��ѧԪ�ء���������Ԫ�ػش��������⡣
|
A |
|
|
|||||||||||||||
|
|
|
|
|
D |
E |
|
|
|
|||||||||
|
G |
|
|
Q |
|
M |
R |
|
||||||||||
|
|
|
|
|
|
|
|
T |
|
|
|
|
|
|
|
|
|
|
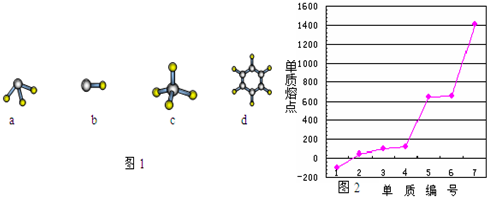
��1��ijԪ��ԭ�ӵĺ�����Ӳ�����������������3������Ԫ�ص�ԭ�ӽṹʾ��ͼΪ ��
 ��2��ijЩԪ�ص�ԭ�ӿ��γ���Ar������ͬ���Ӳ�ṹ�ļ����ӣ���Щ���ӵİ뾶�ɴ�С��˳���ǣ������ӷ��ţ�
��
��2��ijЩԪ�ص�ԭ�ӿ��γ���Ar������ͬ���Ӳ�ṹ�ļ����ӣ���Щ���ӵİ뾶�ɴ�С��˳���ǣ������ӷ��ţ�
��
��3��M��D����Ԫ���γɵĻ����ﺬ�еĻ�ѧ�������� ��������ǣ�����ԡ��Ǽ��ԡ��� ���ӣ�
A�ֱ���D��E��R�γɵķ����У����Ӽ����������ǣ������ʽ�� ��
A��D�γɷ��ӵĿռ�ṹ�����ǣ�����ţ� ����2�֣�