��Ŀ����
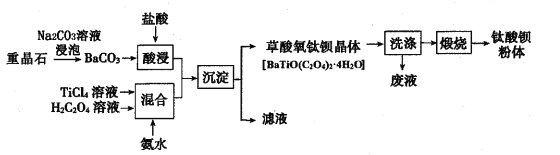
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ[���д�����FeSO4��H2SO4��������Fe2��SO4��3��TiOSO4]��������Ͳ�Ѫ�����������Ĺ���������ͼ��ʾ��

��֪:TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42-��TiO2+ˮ���TiO2��xH2O����Ϊ���淴Ӧ������ṹ��ʽΪCH3CH��OH��COOH��
�ش��������⣺
��1��TiOSO4����Ԫ�صĻ��ϼ���____________��������з�������������Һ�������IJ�����___________��
��2����������Ҫ�ɷ�ΪTiO2��xH2O��������ӷ���ʽ���͵õ�������ԭ��________��
��3��������������Һ�еõ�������������IJ���������____________________�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ______��
��4��������з�����Ӧ�����ӷ���ʽΪ______________________��
��5������ޱ������һ������նȣ�ԭ��������������ˮ�Լ�____________________��
��6��ʵ�����м�����ҺB����Ҫ�����ӵķ�����______________________��
���𰸡�+4 ���� TiO2++��x+1��H2O![]() TiO2xH2O+2H+,��м��H+��Ӧ��c��H+�����ͣ�ƽ��������Ӧ�����ƶ�����ʹTiO2+ת��ΪTiO2xH2O ����Ũ������ȴ�ᾧ������ 1: 4 Fe2++2HCO3-=FeCO3��+H2O+CO2�� ��ֹFe2+������ ȡ������ҺB���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�ʯ����ֽ������˵����ҺB�к���NH4+
TiO2xH2O+2H+,��м��H+��Ӧ��c��H+�����ͣ�ƽ��������Ӧ�����ƶ�����ʹTiO2+ת��ΪTiO2xH2O ����Ũ������ȴ�ᾧ������ 1: 4 Fe2++2HCO3-=FeCO3��+H2O+CO2�� ��ֹFe2+������ ȡ������ҺB���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�ʯ����ֽ������˵����ҺB�к���NH4+
��������
������ͼ��֪����Һ�м�����м��Fe��H2SO4������Fe2��SO4��3��Ӧ����FeSO4����Һ��pH���ٽ�TiOSO4��ˮ�������TiOSO4��ȫˮ������TiO2xH2O���������ˣ�����ΪTiO2xH2O��Fe����ҺΪFeSO4��Һ��FeSO4��Һͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ������������壬��������������ˮ�����յõ���������FeSO4��Һ�м�̼����泥����߷�Ӧ����̼����������������狀Ͷ�����̼��̼�����������������ܽ���������������Һ�Ͷ�����̼������������Һͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ������������塣
��1���ɻ��ϼ۴�����Ϊ0��֪��TiOSO4����Ԫ�صĻ��ϼ���+4�ۣ��������з�������������Һ�������IJ����ǹ��ˣ��ʴ�Ϊ��+4�����ˣ�
��2��TiOSO4����Һ��ˮ������TiO2xH2O��ˮ������ӷ���ʽΪ��TiO2++��x+1��H2O![]() TiO2xH2O+2H+��������м������H+��Ӧ����Һ��c��H+�����ͣ�ˮ��ƽ��������Ӧ�����ƶ�����ʹTiO2+ת��ΪTiO2xH2O�������ʴ�Ϊ��TiO2++��x+1��H2O
TiO2xH2O+2H+��������м������H+��Ӧ����Һ��c��H+�����ͣ�ˮ��ƽ��������Ӧ�����ƶ�����ʹTiO2+ת��ΪTiO2xH2O�������ʴ�Ϊ��TiO2++��x+1��H2O![]() TiO2xH2O+2H+,��м��H+��Ӧ��c��H+�����ͣ�ƽ��������Ӧ�����ƶ�����ʹTiO2+ת��ΪTiO2xH2O��
TiO2xH2O+2H+,��м��H+��Ӧ��c��H+�����ͣ�ƽ��������Ӧ�����ƶ�����ʹTiO2+ת��ΪTiO2xH2O��
��3��FeSO4��Һͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ������������壻���������ڿ��������������������������Ӧ�Ļ�ѧ����ʽΪ4FeSO4+O2 ![]() 2Fe2O3
2Fe2O3
+4SO3������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1��4���ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˣ�1��4��
��4�������ΪFeSO4��Һ�м�̼����泥����߷�Ӧ����̼����������������狀Ͷ�����̼����Ӧ�����ӷ���ʽΪFe2++2HCO3-=FeCO3��+H2O+CO2�����ʴ�Ϊ��Fe2++2HCO3-=FeCO3��+H2O+CO2����
��5�����������ױ��������������������Բ���ޱ������һ������նȣ���������������ˮ���ܷ�ֹFe2+���������ʴ�Ϊ����ֹFe2+��������
��6����ҺBΪ�������Һ��ʵ���Ҽ���笠����ӵķ����ǣ�ȡ������ҺB���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�ʯ����ֽ������˵����ҺB�к���NH4+���ʴ�Ϊ��ȡ������ҺB���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�ʯ����ֽ������˵����ҺB�к���NH4+��

����Ŀ�����ҹ��Ϻ��������������ڴ����Ŀ�ȼ������Ȼ��ˮ����ɱ�ʾΪ![]() ����
����
2017��5�£��й��״κ����ȼ���Բɳɹ���2017��11��3�գ�����Ժ��ʽ������ȼ����Ϊ�¿��֡���ȼ���Ŀ��ɺ����ã��������ڽ���������ٵ���ԴΣ������������һϵ�еĹ�ҵ��Ʒ��
(1)��ij��ȼ���������ж���������ȡһ������Ʒ���ͷų��ļ�����������ۺϳɱ�״����Ϊ166 m3��ʣ�� H2O �����Ϊ0.8m3�������Ʒ�Ļ�ѧʽ�� x=_________________��
(2)��֪�±����ݣ���֪ H2O(l)=H2O(g) ��H=+41![]()
��ѧ�� | C��H | O=O | C=O | H��O |
����/ | 413 | 498 | 803 | 463 |
�ü���ȼ���ȱ�ʾ���Ȼ�ѧ����ʽΪ_____________________________________________________��
(3)����ȼ�ϵ�������ֱ��ȼ�ռ������Ÿ��ߵ�����ת��Ч�ʣ�ij����ȼ�ϵ�أ�����ͨ���������ij�ֽ���������Ϊ���ӵ��壨�������ӿ�Ѩ���ܴ��� O2-�����õ�ظ����ĵ缫��ӦʽΪ__________________________________________��
(4)������ˮ�������������ǹ�ҵ�ϻ����������Ҫ�ֶΡ�������������ˮ��һ�������·�Ӧ����H2��ͬʱ�õ������Ϊ1:3��CO2��CO���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________����������е�CO2����Ũ��ˮ�ѳ���ͬʱ��õ���NH4HCO3���÷�Ӧ�����ӷ���ʽ��_________________________________________________________��