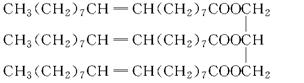��Ŀ����
��ÿ��2�֣���10�֣����꣬����ԭ�ͼ۸������ǣ���ʹ���������Ӵ����������������оƾ������ѽ���ʵ�û��Ρ�
(1)�Ҵ���ͭ�����������������£����Ա������е�����������X��X�ɷ���������Ӧ����д��X��������Һ�����ķ�Ӧ����ʽ(������巴Ӧ����)��______________________________________��
(2)�Ҵ������������ظ������Һ��Ӧ����ֱ��������Y��Y���й����ŵ�������________����Ũ��������¼��ȣ��Ҵ���Y��Ӧ������һ������ζ������W����ѧ����ʽΪ________________________________________��
(3)������ƿ��ɫҺ�壬�ֱ�ʢ��Y��W��ֻ��һ���Լ��Ϳ��Լ��𣬸��Լ�������____________________________________��
(4)�ִ�ʯ�ͻ�������������������ϩ�ܱ������������ɻ�������( )�÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ___________��
)�÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ___________��
(1)CH3CHO��2Ag(NH3)2OH CH3COONH4��2Ag����3NH3��H2O
CH3COONH4��2Ag����3NH3��H2O
(2)�Ȼ���CH3COOH��HOCH2CH3 CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
(3)ʯ��(������������)��
(4)2CH2===CH2��O2 2
2
���������������1���Ҵ���ͭ�����������������£����Ա������е�������������ȩ����ȩ��������Һ����������Ӧ����������李�����������ˮ����ѧ����ʽΪCH3CHO��2Ag(NH3)2OH CH3COONH4��2Ag����3NH3��H2O��
CH3COONH4��2Ag����3NH3��H2O��
��2���Ҵ������������ظ������Һ��Ӧ����ֱ�����������ᣬ����Y�����ᣬ�����Ȼ������ţ��Ҵ������ᷢ��������Ӧ����ѧ����ʽΪCH3COOH��HOCH2CH3 CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��3�����ݣ�2����֪Y�����ᣬW���������������������ԣ���ʹ��ɫʯ����Һ��죬���Լ������ᡢ�����������Լ���ʯ����Һ��
��4��������Ŀ������Ϣ����ϩ��������Ӧ��ԭ��������100%��˵����ϩ��������Ӧֻ���ɻ������飬��ѧ����ʽΪ2CH2===CH2��O2 2
2
���㣺�����Ҵ��Ļ�ѧ���ʣ����ʵ��жϣ���ѧ����ʽ����д

���й����������ʵ������У���ȷ���ǣ� ��
| A���������Ǵ�������ˮ��Һ |
| B�������������Ʒ�Ӧ�ų����� |
| C����������Ա�̼��ǿ�����������Ը�̼������Һ��Ӧ����CO2���� |
| D����������к���̼��˫������������ʹ��ˮ��ɫ |
��14�֣�������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼAװ���Ʊ�����������
��1����ʵ����������ͺ�18O���Ҵ����ã��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
��̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������� ��
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ����Լ� | �Թܢ����Լ� | �л���ĺ��/cm |
| A | 2mL�Ҵ���1mL���ᡢ 1mL18mol?L-1 Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2mL�Ҵ���1mL���� | 0.1 | |
| C | 2mL�Ҵ���1mL���ᡢ 3mL 2mol?L-1H2SO4 | 0.6 | |
| D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʣ�
��3������������90g���Ҵ�138g����������Ӧ�õ�88g�����������Լ���÷�Ӧ�IJ���Ϊ ��
��4��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ��ͼ�У� ����Ϊ�ʵ����Լ����� ����Ϊ�ʵ��ķ��뷽����

���Լ�a�� �����뷽������ �����뷽������ �����뷽�����Ƿ�Һ���ھ��������Ӧ�����Ȼ���ã����ֲ�� �����ţ���
A��ֱ�ӽ����������ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ����������ӷ�Һ©���¿ڷų�
C���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������¿ڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������Ͽڷų�
���ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ���� ��
��5��Ϊ������÷�Ӧ��ס�����λͬѧ�ֱ����������ͼ�мס�������װ�ã���ͬѧ����Ӧ�����ȴ�����ñ���̼������Һ��ȡ��ƿ�еIJ��������Ϊ���������
��15�֣�ij��ѧС���Ա�����Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе������
| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |
��Բ����ƿ�м���12.2 g�������20 mL�״�(�ܶ�ԼΪ0.79 g��cm-3)����С�ļ���3mLŨ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
��Ũ�����������________________________________________ _________��
����Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ
___________________________________________________��
�Ƽ�����λͬѧ�ֱ��������ͼ��ʾ������ʵ���Һϳɱ������ ����װ�� (�г������ͼ�������������ȥ)��

�����л���ķе㣬��ò���______(��ס����ҡ�)װ�á�
������______________________________________________��
����Ҫ��߱���������IJ��ʣ��ɲ�ȡ�Ĵ�ʩ_
______ _������дһ����
��.�ֲ�Ʒ�ľ���
�ȱ���������ֲ�Ʒ���������������״����������ˮ�ȣ���������������ͼ���о��ƣ���������ͼ�з�����������������������ơ�

��ͨ�����㣬����������IJ���Ϊ__________________________________��

 CH3CH2-OH+HR����������ת����ϵ:
CH3CH2-OH+HR����������ת����ϵ: