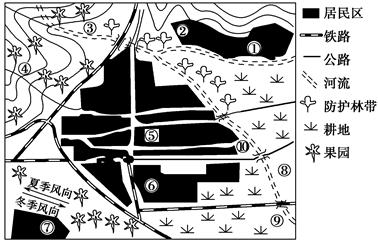
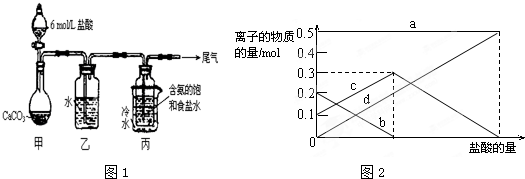
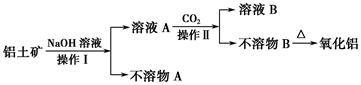
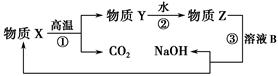
��Ŀ����
������ѧ��ŵ�������Ƴ�һ�ֿ��Խ�ˮ�ֽ�������������Ĵ������⽫ʹ����������Ϊ���ܣ���ʹ̫����ʹ�ò����µ�ʱ��������Ϊ��ˮ���缼�����������й�˵����ȷ���ǣ���������
| A����ˮ���缼������ָˮ�ֽ�����������������ͬʱ�ų����������� |
| B����ˮ���缼����������ת����ʽΪ����ѧ�ܡ����ܡ����� |
| C����ˮ�ֽ������H2��O2�ֱ�ͨ��ȼ�ϵ�ص�������ͨH2�ļ�Ϊ��Դ���� |
| D����ˮ���缼������ʵ�ַ�������е����ŷ� |
D
A��ˮ�ֽ�Ĺ��������ȹ��̣�A˵������B�ˮ���缼����������ת����ʽΪ̫���ܡ���ѧ�ܡ����ܣ�C������ȼ�ϵ���У�ͨH2��Ϊ��Դ������D����ȷ��

��ϰ��ϵ�д�
 �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ