��Ŀ����
ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ�ͼ1��ʾװ����ȡNaHCO3����Ӧ�Ļ�ѧ����ʽΪ��
NH3+CO2+H2O+NaCl=NaHCO3+NH4Cl��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��
��1��װ���ҵ�������______��Ϊ��ֹ��Ⱦ������β���к��е�______��Ҫ�������մ�����
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������______��ϴ�ӡ����գ�
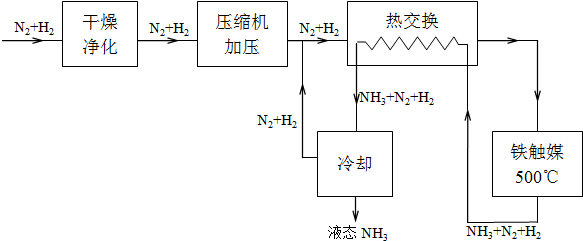
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1min��NaHCO3��Ʒ����ɽ������о���ȡ������t1min��NaHCO3��Ʒ29.6g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��裮��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ2��ʾ��������c��Ӧ����Һ�е�������______�������ӷ��ţ�������Ʒ��NaHCO3��Na2CO3�����ʵ���֮����______��
��4����ȡ10.5gNaHCO3���壬������t1min��ʣ����������Ϊ7.4g������Ѵ�ʣ�����ȫ�����뵽200mL1mol/L�������У����ַ�Ӧ����Һ��H+�����ʵ���Ũ��Ϊ______������Һ����仯���Բ��ƣ���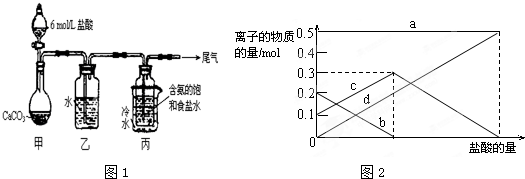
NH3+CO2+H2O+NaCl=NaHCO3+NH4Cl��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��
��1��װ���ҵ�������______��Ϊ��ֹ��Ⱦ������β���к��е�______��Ҫ�������մ�����
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������______��ϴ�ӡ����գ�
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1min��NaHCO3��Ʒ����ɽ������о���ȡ������t1min��NaHCO3��Ʒ29.6g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��裮��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ2��ʾ��������c��Ӧ����Һ�е�������______�������ӷ��ţ�������Ʒ��NaHCO3��Na2CO3�����ʵ���֮����______��
��4����ȡ10.5gNaHCO3���壬������t1min��ʣ����������Ϊ7.4g������Ѵ�ʣ�����ȫ�����뵽200mL1mol/L�������У����ַ�Ӧ����Һ��H+�����ʵ���Ũ��Ϊ______������Һ����仯���Բ��ƣ���

��1��װ�ü����Ʊ�������̼����ķ�Ӧװ�ã����ɵĶ�����̼�����к����Ȼ������壬���Ʊ�̼��������Ӱ�죬װ���ҵ������������Ȼ������壻����β���к��а��������ŷŵ������У���Ҫ����β�����գ�
�ʴ�Ϊ������HCl��NH3��
��2����װ�ñ��в�����NaHCO3�����ķ�ӦΪ��NH3+CO2+H2O+NaCl=NaHCO3��+NH4Cl����ȡNa2CO3ʱ��Ҫ���˵õ����壬ϴ�Ӻ�������յõ�̼���ƣ�
�ʴ�Ϊ�����ˣ�
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1min��NaHCO3��Ʒ����ɽ������о���ȡ������t1min��NaHCO3��Ʒ29.6g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��裮��������ļ��룬������Ӧ CO32-+H+=HCO3-�� HCO3-+H+=CO2��+H2O����Һ���й����ӵ����ʵ����ı仯Ϊ̼������Ӽ�С��̼���������Ũ������̼�������ȫ��ת��Ϊ̼��������ӣ��ٵ��������̼��������ӷ�Ӧ���ɶ�����̼��̼��������Ӽ�С������c���߱�ʾ����̼���������Ũ�ȱ仯��̼�������Ũ��0.2mol/L��̼���������Ũ��Ϊ0.1mol/L����Ʒ��NaHCO3��Na2CO3�����ʵ���֮����1��2��
�ʴ�Ϊ��HCO3-�� 1��2��
��4����ȡ10.5gNaHCO3�������ʵ���=
=0.125mol��������t1min��ʣ����������Ϊ7.4g�����ݻ�ѧ����ʽ���ڵ������仯���㣺
2NaHCO3=Na2CO3+CO2��+H2O��m
21 62
0.1mol 0.05mol 10.5g-7.4g
��Ӧ��NaHCO3���ʵ���=0.125mol-0.1mol=0.025mol��NaHCO3+HCl=NaCl+H2O+CO2���������Ȼ������ʵ���0.025mol��
Na2CO3���ʵ���=0.05mol��Na2CO3+2HCl=2NaCl+H2O+CO2���������Ȼ������ʵ���0.1mol��
ʣ���Ȼ������ʵ���=0.200L��1mol/L-0.025mol-0.1mol=0.075mol��ʣ����Һ��c��H+��=
=0.375mol/L
�ʴ�Ϊ��0.375mol/L
�ʴ�Ϊ������HCl��NH3��
��2����װ�ñ��в�����NaHCO3�����ķ�ӦΪ��NH3+CO2+H2O+NaCl=NaHCO3��+NH4Cl����ȡNa2CO3ʱ��Ҫ���˵õ����壬ϴ�Ӻ�������յõ�̼���ƣ�
�ʴ�Ϊ�����ˣ�
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1min��NaHCO3��Ʒ����ɽ������о���ȡ������t1min��NaHCO3��Ʒ29.6g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��裮��������ļ��룬������Ӧ CO32-+H+=HCO3-�� HCO3-+H+=CO2��+H2O����Һ���й����ӵ����ʵ����ı仯Ϊ̼������Ӽ�С��̼���������Ũ������̼�������ȫ��ת��Ϊ̼��������ӣ��ٵ��������̼��������ӷ�Ӧ���ɶ�����̼��̼��������Ӽ�С������c���߱�ʾ����̼���������Ũ�ȱ仯��̼�������Ũ��0.2mol/L��̼���������Ũ��Ϊ0.1mol/L����Ʒ��NaHCO3��Na2CO3�����ʵ���֮����1��2��
�ʴ�Ϊ��HCO3-�� 1��2��
��4����ȡ10.5gNaHCO3�������ʵ���=
| 10.5g |
| 84g/mol |
2NaHCO3=Na2CO3+CO2��+H2O��m
21 62
0.1mol 0.05mol 10.5g-7.4g
��Ӧ��NaHCO3���ʵ���=0.125mol-0.1mol=0.025mol��NaHCO3+HCl=NaCl+H2O+CO2���������Ȼ������ʵ���0.025mol��
Na2CO3���ʵ���=0.05mol��Na2CO3+2HCl=2NaCl+H2O+CO2���������Ȼ������ʵ���0.1mol��
ʣ���Ȼ������ʵ���=0.200L��1mol/L-0.025mol-0.1mol=0.075mol��ʣ����Һ��c��H+��=
| 0.075mol |
| 0.2L |
�ʴ�Ϊ��0.375mol/L

��ϰ��ϵ�д�
�����Ŀ

 VO2����V3����H2O
VO2����V3����H2O