��Ŀ����
����Ŀ��I.A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ��
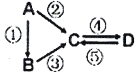
(1)��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�������Ҫ�ɷ֣�д��B-C�Ļ�ѧ����ʽ��_______________��
(2)��D���ʾ������ԣ��ڡ��۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���壬д��A��C�����ӷ���ʽ��______________________��
(3)��A������оƬ���ò��ϣ�BΪA���ʵ������CΪˮ��������Ҫ�ɷ�.д��A��C�����ӷ���ʽ��__________________________��
(4)��A��Ӧ����㷺�Ľ���.�ܷ�Ӧ�õ�A���ڡ��ݷ�Ӧ���õ�ͬһ�ַǽ������ʣ�C����Һ����ʴ��ӡˢͭ��·�壬д��C��D�����ӷ���ʽ��__________________��
(5)��AΪ����ɫ���壬C��D�����������������������Ҫ���ʣ�B��C�ɷ�Ӧ����A��д��B��ȫȼ�յĻ�ѧ����ʽ��__________________________��
���𰸡� 4NH3+5O2![]() 4NO+6H2O 2Al+2OH-+6H2O
4NO+6H2O 2Al+2OH-+6H2O![]() 2[Al(OH)4]-+3H2�� Si+2OH-+H2O=SiO32-+2H2�� Fe+2Fe3+=3Fe2+ 2H2S+3O2
2[Al(OH)4]-+3H2�� Si+2OH-+H2O=SiO32-+2H2�� Fe+2Fe3+=3Fe2+ 2H2S+3O2![]() 2SO2+2H2O
2SO2+2H2O
���������������ƶϣ���1��C��D����ɹ⻯ѧ��������������������ɹ⻯ѧ��������Ҫԭ�����C��D�ǵ������������ת����ϵ��CΪNO,DΪNO2����ΪAΪ���ʣ����AΪN2��BΪNH3��B��C�ǰ��Ĵ�����������ѧ��Ӧ����ʽΪ4NH3��5O2![]() 4NO��6H2O����2����������ЧӦ������ΪCO2��D�����ԣ�������C��CO2��Ӧ���ɣ����DΪAl(OH)3��AΪAl��BΪAl2O3��CΪAlO2����A����C�����ӷ�Ӧ����ʽΪ2Al��2OH����2H2O=2AlO2����3H2������3��A������оƬ���ò��ϣ���AΪSi��BΪA���������BΪSiO2��CΪˮ�����ijɷ֣���CΪNa2SiO3��A����C�����ӷ���ʽSi��2OH����H2O=SiO32����2H2������4��Ӧ����㷺�Ľ�����������AΪFe��C����Һ���ڿ�ʴӡˢͭ��·�壬���CΪFeCl3���ں͢���ͬһ�ַǽ������ʣ��ܷ�Ӧ��Fe�����DΪFeCl2���õ��ķǽ�������ΪCl2����C����D�����ӷ���ʽΪFe��2Fe3��=3Fe2������5��AΪ����ɫ���嵥�ʣ���AΪS��C��D�����������������Ҫ���ʣ���ΪSO2��SO3��Sȼ��ת����SO2����CΪSO2��DΪSO3��SO2��B��Ӧ����S����BΪH2S��H2S��ȫȼ�յĻ�ѧ����ʽΪ��2H2S+3O2
4NO��6H2O����2����������ЧӦ������ΪCO2��D�����ԣ�������C��CO2��Ӧ���ɣ����DΪAl(OH)3��AΪAl��BΪAl2O3��CΪAlO2����A����C�����ӷ�Ӧ����ʽΪ2Al��2OH����2H2O=2AlO2����3H2������3��A������оƬ���ò��ϣ���AΪSi��BΪA���������BΪSiO2��CΪˮ�����ijɷ֣���CΪNa2SiO3��A����C�����ӷ���ʽSi��2OH����H2O=SiO32����2H2������4��Ӧ����㷺�Ľ�����������AΪFe��C����Һ���ڿ�ʴӡˢͭ��·�壬���CΪFeCl3���ں͢���ͬһ�ַǽ������ʣ��ܷ�Ӧ��Fe�����DΪFeCl2���õ��ķǽ�������ΪCl2����C����D�����ӷ���ʽΪFe��2Fe3��=3Fe2������5��AΪ����ɫ���嵥�ʣ���AΪS��C��D�����������������Ҫ���ʣ���ΪSO2��SO3��Sȼ��ת����SO2����CΪSO2��DΪSO3��SO2��B��Ӧ����S����BΪH2S��H2S��ȫȼ�յĻ�ѧ����ʽΪ��2H2S+3O2![]() 2SO2+2H2O��
2SO2+2H2O��