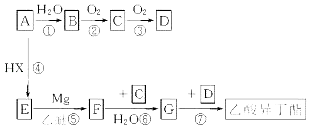
��Ŀ����
����Ŀ����(34Se)����ͬ���壬��Ԫ�ؼ��仯���������彡������ҵ����������ء�ij����С����������(��Ҫ�ɷ���Se������CuSe��Ag2Se������)Ϊԭ�ϣ����������������£�

��ش��������⣺
(1)��ԭ�ӵĴ���������_______,����ͬ��������Ԫ����________(��Ԫ������)��
(2)��֪A��Na2SeO3�����������ƿ�ɽ��,��A�Ļ�ѧ����Ϊ______��C��Na2Se����Na2Se�ĵ���ʽΪ_______��
(3)��������ͼ�е������ڡ�( )�����������Ⱥ�˳��������д��������_____��_____��
(4)д���������ý�̿��ԭB�Ļ�ѧ����ʽ___________________��
(5)��ҺC�������������ӷ���ʽ____________________��
(6)��Na2SeO3��Һ�еμ��Թ��������ᣬ�����ӷ���ʽΪ__________________����֪:Ka1(H2SeO3)=2.7��10-3��Ka2(H2SeO3)=2.5��10-8��Ka(CH3COOH)=1.8��10-5��
(7)�����ɲ����������ķ��������ᴿ,��ô������������Ļӷ��������������¶ȵĹ�ϵ��ͼ��ʾ��

��������п��Ƶ�����¶���________(����)��
A.455�� B.462�� C.475�� D.515��
���𰸡� 18 �顢�� �������� ![]() ���� ���� Na2SeO4+4C
���� ���� Na2SeO4+4C![]() Na2Se+4CO�� 2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-) SeO32-+CH3COOH=HSeO3-+CH3COO- C
Na2Se+4CO�� 2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-) SeO32-+CH3COOH=HSeO3-+CH3COO- C
��������(1). ��֪������ͬ���壬���������Ϊ34����Ԫ�ص��������6�����ӣ�K����2�����ӣ�L����8�����ӣ���M����18�����ӣ�ͬ�������������ڵ�Ԫ��������壬�ʴ�Ϊ��18���顢�壻
(2). ���Na2SO3�Ļ�ѧ�������������ƿ�֪��Na2SeO3�Ļ�ѧ�������������ƣ�SeԪ���������6�����ӣ��õ�2��Naԭ��ʧȥ��2�������γ��ȶ��ṹ��Na2Se�ĵ���ʽΪ��![]() ���ʴ�Ϊ���������ƣ�
���ʴ�Ϊ���������ƣ�![]() ��
��
(3). ��ˮ��֮ǰӦ���ս��Ĺ�����飬����߽����ʣ����ݡ�Cu��Ag����������ҺA�����ж�ˮ����IJ����ǹ��ˣ��ʴ�Ϊ�����飻���ˣ�
(4). A��Na2SeO3��ͨ����������ɣ��õ��Ĺ���B��Na2SeO4���������ý�̿��ԭNa2SeO4�õ�Na2Se��CO�����ݵ�ʧ�����غ��ԭ���غ㣬�÷�Ӧ�Ļ�ѧ����ʽΪ��Na2SeO4+4C![]() Na2Se+4CO�����ʴ�Ϊ��Na2SeO4+4C
Na2Se+4CO�����ʴ�Ϊ��Na2SeO4+4C![]() Na2Se+4CO����
Na2Se+4CO����
(5). ����ҺC��ͨ������������ɰѻ�ԭ��ǿ��Se2-����Ϊ����Se��ͨ��CO2���Լ�����Ӧ����Һ�ļ��ԣ�������Se���������������֪�÷�Ӧ�����ӷ���ʽΪ��2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-)���ʴ�Ϊ��2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-)��
(6). ��H2SeO3��CH3COOH�ĵ��볣����֪������ǿ����˳��Ϊ��H2SeO3��CH3COOH��HSeO3��������Na2SeO3��Һ�еμ��Թ��������ᣬ��Ӧ����HSeO3����CH3COO�������ӷ���ʽΪ��SeO32-+CH3COOH=HSeO3��+CH3COO�����ʴ�Ϊ��SeO32-+CH3COOH=HSeO3��+CH3COO����
(7). ��ͼ��֪����475��ʱ�������Ļӷ���������������������ѡ��475�棬�ʴ�Ϊ��C��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�