��Ŀ����
14������98%��Ũ���ᣨ�ܶ�Ϊ1.84g•cm-3�����Ƴ�Ũ��Ϊ0.5mol•L-1��ϡ����500ml����1��ѡ�õ���Ҫ�����У��ٲ����������ձ�������Ͳ���ܽ�ͷ�ιܣ���500mL����ƿ��
��2���뽫���и�����������ȷ��������ں����ϣ�
A������Ͳ��ȡŨH2SO4 B�������ߵ�ҡ��
C���ý�ͷ�ιܼ�����ˮ���̶��� D��ϴ����������
E��ϡ��ŨH2SO4 F������Һת������ƿ
�������ȷ��˳������ΪAEFCBD��
��3����Ҫ�ش��������⣺
������Ũ��������Ϊ13.6ml��
����ת������ƿǰ�ձ���Һ��Ӧ��ȴ�������ʹŨ��ƫ�ߣ���ϴ���ձ��Ͳ�����2��3�Σ�ϴ��ҺҲҪת������ƿ�������ʹŨ��ƫ�ͣ�
�۶���ʱ����ʹ��Һ��Һ����̶������У������ӻ�ʹŨ��ƫ�ߣ�
���� ��1���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2����������һ�����ʵ���Ũ����Һ��һ�㲽������
��3������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�������Һϡ�������������ʵ����ʵ������������ҪŨ����������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1�����������м��㡢��ȡ��ϡ�͡�ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡŨ���ᣬ���ձ���ϡ�ͣ�������Ͳ��ȡˮ�����ձ��������ò��������裮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ��
��2����Ũ��������ϡ�����һ�㲽�裺���㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������ȷ��˳��Ϊ��AEFCBD��
�ʴ�Ϊ��AEFCBD��
��3����98%��Ũ���ᣨ�ܶ�Ϊ1.84g•cm-3�������ʵ���Ũ��C=$\frac{1000��1.84��98%}{98}$=18.4mol/L������ҪŨ�������ΪV��������Һϡ�������������ʵ����ʵ���������V��18.4mol/L=500ml��0.5mol•L-1�����V=13.6mL��
�ʴ�Ϊ��13.6��
������ƿΪ��������������ʢ�Ź���Һ�壬��ת������ƿǰ�ձ���Һ��Ӧ��ȴ��������ȶ��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���ϴ���ձ��Ͳ�����2��3�Σ�ϴ��ҺҲҪת������ƿ������ᵼ�����ʵ����ʵ���ƫС��������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ����ȴ��ƫ�ߣ�ƫ�ͣ�
�۶���ʱ����ʹ��Һ��Һ����̶������У������ӻ�ʹŨ�ȣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ��������������Ŀ�ѶȲ����ؿ���ѧ������ʵ��������

��FeCl3 ��FeCl2 ��Fe��OH��3 ��CuS��
| A�� | ֻ�Тڢ� | B�� | ֻ�Т٢� | C�� | �٢ڢۢ��ܵõ� | D�� | �٢ڢ��ܵõ� |
| A�� | �ߴ��ȵĹ赥�ʹ㷺�����������ά | |
| B�� | �ƿ�����ұ�������� | |
| C�� | С�մ����������θ����� | |
| D�� | �������ƿ�����DZˮͧ��������Դ |
| A�� | pH=3��̼���У�c��H+���T3.0 mol•L-1 | |
| B�� | ������������Һ�У�c��Na+���Tc��CH3COO-�� | |
| C�� | pH=13���ռ���Һ�У�c��OH-��=1.0��10-1 mol•L-1 | |
| D�� | pHΪ2�������У�c��H+���Tc��Cl-��+c��OH-�� |
| A�� | ͭ�ܵ��磬����ͭ�ǵ���� | |
| B�� | ��̬�Ȼ��Ʋ����磬���Ȼ����ǵ���� | |
| C�� | �Ȼ���ˮ��Һ�ܵ��磬�����Ȼ���ˮ��Һ�ǵ���� | |
| D�� | ������������ˮ�ܵ��磬���ԣ����������ǵ���� |
����˵����ȷ���ǣ�������
| A�� | �������ͻ�ԭ�������ʵ���֮��Ϊ4��1 | |
| B�� | Cl₂���������������ǻ�ԭ���� | |
| C�� | ת��2mol����ʱ���õ���״���µ�����Ϊ11.2L | |
| D�� | �÷�Ӧ�����ӷ���ʽ��MnO2+4H++2Cl-=Mn2++Cl2��+2H2O |
| A�� | H2CO3?2H++CO32- | B�� | NH3•H2O?NH4++OH- | ||
| C�� | Fe��OH��3=Fe3++3OH- | D�� | NaHSO4$\frac{\underline{\;����\;}}{\;}$Na++H++SO42- |
| A�� | ��������ѹǿ���� | B�� | ���������ܶȱ�С | ||
| C�� | ÿ����2molB��ͬʱ����1molC | D�� | C��D��Ũ�ȱȲ��� |
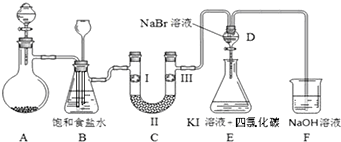
��1���Ʊ�����ѡ�õ�ҩƷΪ�������̺�Ũ���ᣬ����صĻ�ѧ��Ӧ����ʽΪ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
��2��װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���d��
| a | b | c | d | |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | ��ˮCaCl2 | Ũ���� | ��ˮCaCl2 |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��5��װ��F��������NaOH��Һ����ʣ�����������д����Ӧ�Ļ�ѧ����ʽ��Cl2+2NaOH=NaCl+NaClO+H2O��