��Ŀ����
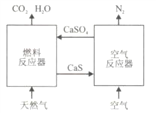
����Ŀ����Դ����ʯ�ͻ�����ս��Ŀ�꣬��չ��CH4��CO2��Ϊԭ�ϵġ�C1��ѧ����Ϊ���������ı�Ȼ���ơ�ͨ����Ȼ���к���H2S���ж����壬����Ϊ��Ȼ���ϳɰ��Ĺ������̡�
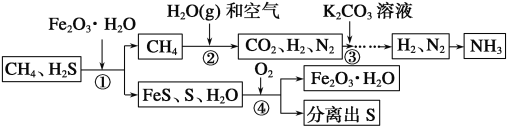
(1)�ϳɰ���ԭ��֮һΪ��������������Ϊ�ϳɰ��ṩ������������__________��
(2)�ٴ�����Fe2O3��H2O��Ŀ����__________��
(3)�ڴ�CH4��H2O(g)��Ӧ����CO2��H2����Ӧ�Ļ�ѧ����ʽ��_________________________��
(4)�۴�һ�����K2CO3��Һ������CO2��K2CO3��Һ��CO2��Ӧ����̼�����(KHCO3)���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
(5)�������в���ѭ����������__________��
���𰸡�(1)���� (2)��ȥH2S
(3)CH4��2H2O(g)===CO2��4H2
(4)K2CO3��CO2��H2O===2KHCO3
(5)Fe2O3��H2O
��������(1)������ͼ�ɿ�����Ϊ�ϳɰ��ṩ�����������ǿ�����(2)��Ȼ���к��е�H2S�������ж������壬�����ŷŵ������У�ͨ������Fe2O3��H2O������������ȥ��(3)CH4��H2O(��)��Ӧ����CO2��H2�����������غ㶨�ɣ���Ӧ�Ļ�ѧ����ʽ��CH4��2H2O(g)===CO2��4H2��(4)K2CO3��Һ��CO2��Ӧ����̼�����(KHCO3)�����������غ㶨�ɣ���Ӧ�Ļ�ѧ����ʽ��K2CO3��CO2��H2O===2KHCO3��(5)�ڸ������м�����Fe2O3��H2O�������������Fe2O3��H2O�����Ը������в���ѭ����������Fe2O3��H2O��

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
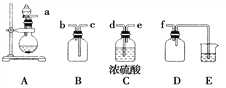
��ٽ������½������������ϵ�д�����Ŀ�����������ʾۼ�״̬����Ҫ��ʽ֮һ����ʵ���һ�ҵ�Ͼ������Ʊ����塣������ijͬѧ��Ƶ�ʵ�����Ʊ�������һЩװ�á�
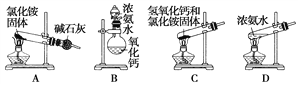
(1)������ʵ������ȡ������װ�ú�ѡ�õ��Լ������в��ܵõ���������________(����ĸ)��
(2)����װ��B�������ṩ���Լ������Ʊ�������������________________(������Ļ�ѧʽ)��
���� | �����Լ� |
SO2 | NaHSO3(��)��H2SO4(Ũ) |
Cl2 | MnO2(��)��HCl(Ũ) |
H2S | Na2S(��)��H2SO4(Ũ) |
O2 | Na2O2 (��)��H2O |
(3)ʵ�����Ʊ����ռ������NO2������������ͼ��ʾ���������������Ӹ������ӿڣ�˳��Ϊa��________��________��________��________��f��װ��D��������__________________________��װ��E��ʢ�ŵ���Һ��________���Ʊ�ʱ����ƿ��װ����ͭƬ���ӷ�Һ©������ƿ�еμ�Ũ���ᣬ��ƿ�е�������__________________________________________________________________��