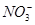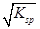��Ŀ����
ij��ɫ��Һ��ֻ��������NH4����K����Al3����Mg2����HCO3����Cl����I����MnO4����SO42���������еļ������ӡ���������ɣ��ֽ�������ʵ�飺
��ȡl0mL����Һ���Թ��еμ�������Ba(NO3��2��Һ����ϡ�����ữ����˵õ�0.03mol��ɫ�����ס�
��ȡ������Ӧ�����Һ������AgNO3��Һδ������������
����ȡl0mL����Һ���Թ��У��μ�NaOH��Һ������ɫ�����ң�������NaOH�����ʵ���Ϊ0. 03 molʱ�����������ﵽ������μ�NaOH��Һ�����ȣ���ʼ������������ռ���������������ɱ����Ϊ0. 224L(�����ȫ���ݳ�������������ȫ�ܽ⡣�����ƶ���ȷ����
��ȡl0mL����Һ���Թ��еμ�������Ba(NO3��2��Һ����ϡ�����ữ����˵õ�0.03mol��ɫ�����ס�
��ȡ������Ӧ�����Һ������AgNO3��Һδ������������
����ȡl0mL����Һ���Թ��У��μ�NaOH��Һ������ɫ�����ң�������NaOH�����ʵ���Ϊ0. 03 molʱ�����������ﵽ������μ�NaOH��Һ�����ȣ���ʼ������������ռ���������������ɱ����Ϊ0. 224L(�����ȫ���ݳ�������������ȫ�ܽ⡣�����ƶ���ȷ����
| A���϶���K+��Al3+��Mg2+��SO42- |
| B���϶���K+��NH4+��Al3+��SO42�� |
| C���϶�û��K+��HCO3-��MnO4- |
| D���϶�û��K+��NH4+��Cl- |
B
�����������Һ����ɫ�ģ���һ��������MnO4�������ݢٿ�֪��ɫ���������ᱵ�����ԭ��Һ�к���0.03molSO42�������ݢڿ�֪ԭ��Һ�в�����Cl����I������ȡl0mL����Һ���Թ��У��μ�NaOH��Һ������ɫ�����ң�������NaOH�����ʵ���Ϊ0. 03 molʱ�����������ﵽ������μ�NaOH��Һ�����ȣ���ʼ������������ռ���������������ɱ����Ϊ0. 224L(�����ȫ���ݳ�������������ȫ�ܽ⣬��˵����ɫ����һ��������������������0.01mol�����ӣ���˲�����Mg2����HCO3���������ǰ��������ʵ�����0.01mol��˵��ԭ��Һ�к���0.01mol NH4����������Һ�ĵ����Կ�֪0.03mol��2��0.01mol��3+0.01mol��������Һ��һ�������м����ӣ���˴�ѡB��

��ϰ��ϵ�д�
�����Ŀ
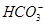 )��0.1 mol��L��1����Һ�У�
)��0.1 mol��L��1����Һ�У�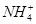 ��Al3����Cl����
��Al3����Cl����