��Ŀ����
��״���£���a L SO2��Cl2��ɵĻ������ͨ��100 mL 0.2 mol��L��1Fe2(SO4)3��Һ�У���ַ�Ӧ����Һ���ػ�ɫ��dz����Ӧ�����Һ�м���������BaCl2��Һ�������ó������ˡ�ϴ�ӡ��������أ�������Ϊ23.3 g�������й��ڸù��̵��ƶϲ���ȷ����
| A������������������ʵ���Ϊ0.04 mol |
| B�����������SO2���ʵ���Ϊ0.04 mol |
| C�����õij���Ϊ0.1 mol BaSO4 |
| D��a��ȡֵ��ΧΪ1.344< a <1.792 |
A
�����������a L SO2��Cl2��ɵĻ������ͨ��100 mL 0��2 mol��L��1Fe2(SO4)3��Һ�У���ַ�Ӧ����Һ���ػ�ɫ��dz����Ҫ�����ˣ�1������������������ˮ��Һ�е�������ԭ��Ӧ�����߰����ʵ���֮��1:1���У�2��������Һ���ػ�ɫ��dz��֪ǰ�߷�Ӧ�������������̶������˶������������������ӵ�������ԭ��Ӧ���Ҷ��߰����ʵ���֮��1:2���С��پ���Ӧ�����Һ�м���������BaCl2��Һ�������ó������ˡ�ϴ�ӡ��������أ�������Ϊ23.3 g��֪����Ϊ���ᱵ����
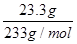 ��0.1Ħ������C��ȷ��ͬʱ֪��Һ�����������Ϊ0.1Ħ��������������������0��06Ħ������ô����0��04Ħ��������϶������ڶ����������������ɴ���֪��0.04Ħ������������������B��ȷ������Һ�����е�����������ȫ������ԭʱ������0.04Ħ������������Ҫ��������0.02Ħ������ô����0.02Ħ��������������˺������ķ�Ӧ������������0.02Ħ������A������������a L�����������ʵ���Ϊ0.08molʱ����1.792��ʱ��Ӧǡ����ɣ�ֻҪ�����������0��06Ħ������������������1��344��ʱ�������⣬D��ȷ��
��0.1Ħ������C��ȷ��ͬʱ֪��Һ�����������Ϊ0.1Ħ��������������������0��06Ħ������ô����0��04Ħ��������϶������ڶ����������������ɴ���֪��0.04Ħ������������������B��ȷ������Һ�����е�����������ȫ������ԭʱ������0.04Ħ������������Ҫ��������0.02Ħ������ô����0.02Ħ��������������˺������ķ�Ӧ������������0.02Ħ������A������������a L�����������ʵ���Ϊ0.08molʱ����1.792��ʱ��Ӧǡ����ɣ�ֻҪ�����������0��06Ħ������������������1��344��ʱ�������⣬D��ȷ��
��ϰ��ϵ�д�
�����Ŀ
 2ClO2����K2SO4��2CO2����2H2O
2ClO2����K2SO4��2CO2����2H2O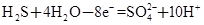 ��
��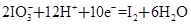
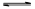 KI3
KI3