��Ŀ����
����Ŀ������˵���������
A.�к��Ȳⶨʵ������Ҫ�IJ��������У��ձ�����Ͳ���¶ȼơ����β��������
B.��ϩ��ȼ������1411.3kJ��mol��1������ϩȼ�յ��Ȼ�ѧ����ʽΪ��C2H4(g)+3O2(g)��2CO2(g)+2H2O(g)��H����1411.3kJ��mol��1
C.P(s������)��P(s������)����H����39.3kJ��mol��1��P(s������)��P(s������)����H����17.6kJ��mol��1�ɴ���֪�����ȶ��������Ǻ���
D.MgCO3�ֽ��������ϵ��ͼ��ʾ��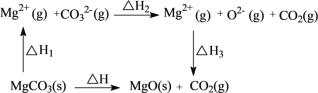 ����H����H1+��H2+��H3
����H����H1+��H2+��H3
���𰸡�B
��������
A. �к��Ȳⶨʵ������Ҫ�IJ��������У���С�ձ�����Ͳ���¶ȼơ����β���������������ⲻ����A����
B. ��ϩ��ȼ������1411.3kJ��mol��1��Ϊ1mol��ȼ����ȫȼ�������ȶ���������ʱ�ͷŵ�����������ϩȼ�յ��Ȼ�ѧ����ʽΪ��C2H4(g)+3O2(g)��2CO2(g)+2H2O(l)��H����1411.3kJ��mol��1�� �������⣬B��ȷ��
C. P(s������)��P(s������)����H����39.3kJ��mol��1��P(s������)��P(s������)����H����17.6kJ��mol��1�ɴ���֪�������е�������ͣ������ȶ��������Ǻ��ף������ⲻ����C����
D. ����MgCO3�ֽ��������ϵͼ������H����H1+��H2+��H3�������ⲻ����D����
��ΪB��

����Ŀ����������Ⱦ����Դ��ȱ��������ͻ������Դ��ѭ��������Ϊ��Ҫ����ҵ �����ú� Cu2O�������� Al2O3��Fe2O3 �� SiO2���Ŀ�����ȡͭ�Ĺ����������£�
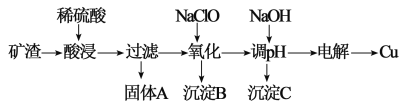
��֪����Cu2O ��������������ת���ɵ��� Cu �ͺ� Cu2+����Һ��
�ڼ��������������ʱ�� pH ���±���
�������� | Al(OH)3 | Fe(OH)2 | Fe(OH)3 | Cu(OH)2 |
����ʱ�� pH | 4.0~5.2 | 5.8~8.8 | 1.1~3.2 | 5.4~6.7 |
�ش��������⣺
��1���������� A ���еijɷ�Ϊ Cu ����һ�����ʣ��ӹ��� A �з���� Cu �Ŀ� ���Լ�Ϊ_____��Һ���ѧʽ����
��2����������������ˡ�ϴ�Ӻ���Ԫ�ص���Ҫ������ʽΪ__________�����ӷ��ţ�����������ӵij��û�ѧ�Լ���_________________��
��3���� NaClO ������������Һ�� pH Ϊ_____���õ����� B ��һ�־���Ư�� �Ե����� D���÷�Ӧ���ӷ���ʽΪ_________��
��4��25��ʱ���� NaOH ���������Һ pH �õ� Al(OH)3 �������� pH=5.3 ʱ������ ��Һ�� c(Al3+)=_____����֪ 25��ʱ Ksp[Al(OH)3]=1.3��10-33��
��5���ö��Ե缫����ý���ͭʱ����ʼ������������������������ʵ���֮��Ϊ___________��