��Ŀ����
����Ŀ�������ס����ǵ�VA���Ԫ�أ����ǵĵ��ʼ��仯���������������о�����Ҫ���á���ش���������:
(1) ��̬��ԭ�ӵ����������Ų�ʽΪ______����_____���ܼ���
(2) N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________��
(3) ��Ԫ�ص�һ����Ҫ����������CO(NH2)2�����У��Ҽ��ͦм�����Ŀ֮��Ϊ______��
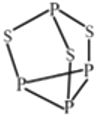
(4) P4S3 ����������������ӽṹ��ͼ��ʾ���ж�P4S3 ��������ԭ�ӵ��ӻ��������Ϊ_____��ÿ��P4S3 �����к��µ��ӶԵ���ĿΪ_______��
(5) Na3AsO4 ������ɱ�����AsO43-�Ŀռ乹��Ϊ_______����AsO43-��Ϊ�ȵ������һ�ַ���Ϊ_____ (��д��ѧʽ)��
(6) ���黯��(GaAs) Ϊ�ڻ�ɫ���壬�۵�Ϊ1238�棬�侧���ṹ��ͼ��ʾ���þ�������____���壬��֮����ڵ���������_______��

�� �黯�ؾ����о���Gaԭ�ӵȾ��������Gaԭ����______������֪�黯�ؾ����߳�Ϊapm�����ܶ�Ϊ��g��cm-3,���ӵ�������ֵ_______(�г�����ʽ����)��
���𰸡� 4s24p3 8 N>P>As 7:1 sp3 10 ���������� CCl4 ԭ�� ���ۼ� 12 ![]()
�����������������������Ҫ��������Ų�ʽ����д����һ�����ܴ�С�ıȽϡ��Ҽ��ͦм��ļ��㡢�ӻ���ʽ�Ϳռ乹�͵��жϡ��ȵ��������д���������ͺ��������������ж��������ķ����ͼ��㡣
��1��Asλ��Ԫ�����ڱ��е�������VA�壬As��ԭ������Ϊ33��Asԭ�Ӻ�����33�����ӣ����ݹ���ԭ������̬Asԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3����̬Asԭ�ӵ����������Ų�ʽΪ4s24p3������1s��2s��2p��3s��3p��3d��4s��4p�ܼ�����8���ܼ���
��2��ͬ������ϵ��µ�һ��������С��N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��ΪN![]() P
P![]() As��
As��
��3�����صĽṹʽΪ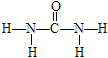 ��1��CO��NH2��2�����к�7���Ҽ���1���м����Ҽ��ͦм�����Ŀ֮��Ϊ7:1��
��1��CO��NH2��2�����к�7���Ҽ���1���м����Ҽ��ͦм�����Ŀ֮��Ϊ7:1��
��4������P4S3�Ľṹ��ÿ��Sԭ���γ�2��P-S����ÿ��Sԭ���γ�2���Ҽ���S�ϻ������Թµ��Ӷԣ�Sԭ��Ϊsp3�ӻ���ÿ��Sԭ���������Թµ��Ӷԣ�ÿ��Pԭ������һ�Թµ��Ӷԣ�ÿ��P4S3�����к��µ��ӶԵ���ĿΪ3![]() 2+4
2+4![]() 1=10��
1=10��
��5��AsO43-������ԭ��As�ŵ��Ӷ���Ϊ![]() ��5+3-4
��5+3-4![]() 2��=0���ɼ����Ӷ���Ϊ4���۲���Ӷ���Ϊ0+4=4��VSEPRģ��Ϊ���������ͣ�����As��û�йµ��Ӷԣ�AsO43-�Ŀռ乹��Ϊ���������͡������������AsO43-��Ϊ�ȵ�����ķ���ΪSiF4��CCl4����
2��=0���ɼ����Ӷ���Ϊ4���۲���Ӷ���Ϊ0+4=4��VSEPRģ��Ϊ���������ͣ�����As��û�йµ��Ӷԣ�AsO43-�Ŀռ乹��Ϊ���������͡������������AsO43-��Ϊ�ȵ�����ķ���ΪSiF4��CCl4����
��6����GaAs���۵�ϸߣ�ԭ�Ӽ��γɹ��ۼ���GaAs��������ԭ�Ӿ�������֮����ڵ��������ǹ��ۼ���
������GaAs�ľ���������Gaԭ�ӹ������������ṹ������Gaԭ�ӵȾ��������Gaԭ����12����GaAs�����߳�Ϊapm�����������Ϊ��apm��3=��a![]() 10-10��3cm3��������̯�����������к�Ga��8
10-10��3cm3��������̯�����������к�Ga��8![]() +6
+6![]() =4����As��4����1mol��������Ϊ
=4����As��4����1mol��������Ϊ![]() 10-30
10-30![]() NAcm3��1mol���������Ϊ
NAcm3��1mol���������Ϊ![]() 10-30
10-30![]() NAcm3
NAcm3![]() ��g/cm3=145g�������ӵ�����NA=
��g/cm3=145g�������ӵ�����NA=![]() 1030��
1030��

����Ŀ��2017��12��19�գ��й���ɫ̼������ӡ�����������轭���ء�̼����С��ƺŵľ����������н����س�Ϊȫ������������ʡ����̼����С���̼�㣬��ָͨ��ֲ�����֡�ɭ�ֹ�����ֲ���ָ��ȴ�ʩ������ֲ�����������մ����еĶ�����̼6CO2(g) +6H2O(l) === C6H12O6(s)+6O2(g) ��������̶���ֲ���������У��Ӷ��������������ڴ�����Ũ�ȵĹ��̡������ơ���֪ÿ����1molCO2��Ҫ��������ԼΪ470kJ.�ݴ˻ش��������⣺
��1��̼�������������ת����ʽ��__________��ת��Ϊ___________�ܣ���������ϱ�����ÿ1m3��ľ,��Լ����������Ϊ1.88��107kJ����Լ��������CO2______�֣�������ȼ�յ��Ȼ�ѧ����ʽΪ��_____________ ��
��2����ҵ�����е�CO2���ü�Һ���ա��������ķ�Ӧ���£�
CO2(g)��2NaOH(aq)===Na2CO3(aq)��H2O(l) ��H����a kJ��mol��1
CO2(g)��NaOH(aq)===NaHCO3(aq) ��H����b kJ��mol��1
�ٷ�ӦCO2(g)��H2O(l)��Na2CO3(aq)===2NaHCO3(aq)�Ħ�H��______ kJ��mol��1(�ú�a��b�Ĵ���ʽ��ʾ)��
��3������Һ����ʾ���Ĺ�����ʹ�õĻ�ѧ��ϴ��NF3��CO2һ����һ���������� ,�ڴ����е������ɳ���740��֮�á������Ǽ��ֻ�ѧ���ļ���:
��ѧ�� | N��N | F��F | N��F |
����/kJ��mol-1 | 941.7 | 154.8 | 283.0 |
�ٷ�Ӧ:N2(g)+3F2(g)��2NF3(g)��H=_________________
������˵���в���ȷ����_____________
A.����:N2(g)![]() 2N�ų�����
2N�ų�����
B.����:N+3F![]() NF3(g)�ų�����
NF3(g)�ų�����
C.ʹ�ô����ܼ�С��Ӧ�Ħ�H
