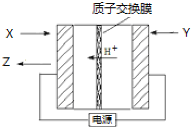
��Ŀ����
����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
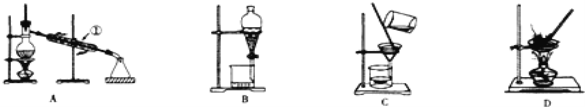
(1)���Ȼ�����Һ�еõ��Ȼ��ع��壬ѡ��װ��______![]()
![]() �����װ��ͼ����ĸ����ͬ
�����װ��ͼ����ĸ����ͬ![]()
![]() ����ȥ����ˮ�е�Cl_�����ʣ�ѡ��װ��______��
����ȥ����ˮ�е�Cl_�����ʣ�ѡ��װ��______��
(2)�ӵ�ˮ�з����I2�ķ��뷽��������Ϊ______��
(3)װ��A�Тٵ�������______����ˮ�ķ����Ǵ�______�ڽ�ˮ![]() ��
��
(4)��ˮ���̲��ŷḻ����Դ����ʵ������ȡ������ˮ�������������̵�ʵ�飺
![]()
�����к�Fe3+��Mg2+��Ca2+��SO42-�����ʣ���Ҫ�ᴿ������ۺ����á������ᴿ�IJ����У��ټ��������Na2CO3��Һ���ڼ������BaCl2��![]() ��Һ���ۼ��������NaOH��Һ���ܵ�����Һ��pH����7�����ܽ⣻���ˣ�����������ȷ�IJ���˳����______(��ѡ����ĸ) ��
��Һ���ۼ��������NaOH��Һ���ܵ�����Һ��pH����7�����ܽ⣻���ˣ�����������ȷ�IJ���˳����______(��ѡ����ĸ) ��
A.�ݢڢۢ٢ޢܢ� B.�ݢ٢ڢۢޢܢ� C. �ݢڢ٢ۢܢޢ� D.�ݢۢڢ٢ܢޢ�
���𰸡�D A ��ȡ��Һ ������ �¿� A
��������
��1�����Ȼ�����Һ�еõ��Ȼ��ع��壬Ӧ�������ķ�������ȥ����ˮ�е�Cl-��������ȡ����ˮ����������ķ�����
��2�������л��ܼ��е��ܽ�Ƚϴ�����ȡ��Һ�ķ������룻
��3�����������Ľṹ�ص����;��ȷ�����������ƣ�����������ȴˮӦ���¿ڽ��룬�Ͽ�������
��4��������ת��Ϊ�����������ȥ������������̼�������þ����������������Fe3+������������������ñ����ӣ�Ҫע������ʵ�˳��ӵ��Լ�����ܰ�ǰ���ȼӵĹ����Լ�������
��1���Ȼ�����Һ�еõ��Ȼ��ع��壬Ӧ�������ķ�������ȥ����ˮ�е�Cl-��������ȡ����ˮ����������ķ�����
�ʴ�Ϊ��D��A��
��2�������л��ܼ��е��ܽ�Ƚϴ�����ȡ��Һ�ķ���������
�ʴ�Ϊ����ȡ��Һ��
��3��װ��A�Тٵ������������ܣ�����������ȴˮӦ���¿ڽ��룬�Ͽ�������
�ʴ�Ϊ�������ܣ��¿ڣ�
��4����ȥ�����еĿ��������ʣ�Fe3+��Mg2+��Ca2+��SO42-ʱ�����Լ������NaOH��ȥ��þ���Ӻ������ӣ���Mg2++2OH-=Mg��OH��2����Fe3++3OH-�TFe��OH��3����
�������BaCl2��ȥ����������ӣ���SO42-+Ba2+=BaSO4�����������Na2CO3��ȥ�������ӵĶ���ı����ӣ���Ca2++CO32-=CaCO3����̼���Ʊ�������Ȼ���֮���������ƺ��Ȼ������Եߵ������˳��Ȼ�������Һ��pH����7���������ɣ���˳��Ϊ�ݢڢۢ٢ޢܢߣ��ʴ�Ϊ��A��

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�����Ŀ�������������ۺϴ�����Ӧ�ü����ǿ�����Ա����Ҫ�о�����,CO��SO2��NO2����Ҫ�Ĵ�����Ⱦ���塣
��1���������CO����ȡ������Դ������(CH3OCH3)��ԭ�ϡ�
��֪��CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H1=-41.0 kJ/mol
CO2(g)+H2(g) ��H1=-41.0 kJ/mol
��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H2=-49.0 kJ/mol
CH3OH(g)+H2O(g) ��H2=-49.0 kJ/mol
��CH3OCH3(g)+H2O(g)![]() 2CH3OH(g) ��H3=+23.5 kJ/mol
2CH3OH(g) ��H3=+23.5 kJ/mol
��Ӧ2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g)����H=________.
CH3OCH3(g)+H2O(g)����H=________.
��2����֪973 Kʱ,SO: ��NO2 ��Ӧ����SO,��NO,��������徭�����������SO,�������Ʊ����ᡣ
��973 Kʱ,���:
NO2(g)![]() NO(g)+ 1/2O2(g) K1=0.018;
NO(g)+ 1/2O2(g) K1=0.018;
SO2(g)+1/2O2(g)![]() SO3(g) K2=20;
SO3(g) K2=20;
��ӦSO2(g)+NO2(g)![]() SO3(g)+NO(g)��K3=________
SO3(g)+NO(g)��K3=________
��973Kʱ,���ݻ�Ϊ2 L���ܱ������г���SO2��NO2 ��0.2mol��ƽ��ʱSO2��ת����Ϊ________��
�ۺ�ѹ��,SO2�ķ�ѹPSO2���¶ȵı仯��ͼ��ʾ:
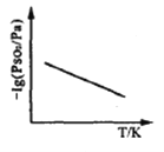
���¶�����ʱ,SO2(g)+NO2(g)![]() SO3(g)+NO(g)�Ļ�ѧƽ�ⳣ��_____(����������������С��)�� �ж����� ��_________.
SO3(g)+NO(g)�Ļ�ѧƽ�ⳣ��_____(����������������С��)�� �ж����� ��_________.
��3������������ȥ����ˮ�е�NO3-��
������������ˮ��NO3-��Ӧ�����ӷ���ʽΪ4Fe+NO3-+10H+=4Fe2++NH4++3H2O���о�����,��PHƫ�ͽ��ᵼ��NO3-��ȥ�����½�,��ԭ����___________.
����ͬ������,��������ȥ����ͬˮ���е�NO3-�������нϴ���졣
�±������������������ԭ�������____;����0~20min,��NO3-��ʾ��ƽ����Ӧ����Ϊ____mol��L-l��min-1��
��Ӧʱ��/min | 0 | 10 | 20 | 30 | 40 | |
�� | c(NO3-)/10-4 mol/L | 8 | 3.2 | 1.6 | 0.8 | 0.64 |
�� | c(NO3-)/10-4 mol/L (������Cu2+) | 8 | 0.48 | 0.32 | 0.32 | 0.32 |
��4����NaOH��Һ����SO2�ɵ�NaHSO3��Һ,��NaHSO3��Һ�и�����Ũ�ȵĹ�ϵ�����з�������������___��(��֪������K1(H2SO3)=1.5��10-2��,K2(H2SO3)=1.02��10-7)
A.[Na+]+[H+]=[HSO3-]+2[SO32-]+[OH-]
B.[Na+]=[HSO3-]+[SO32-]+[H2SO3]
C.[Na+]>[SO32-]>[HSO3-]>[OH-]>[H+]
D.[H+]+[SO32-]=[OH-]+[H2SO3]