��Ŀ����
����Լռ����������71%����һ��Զδ��ȫ�����ľ�ѧ��Դ���⣬��ˮˮ��Դ�����úͺ�ˮ��ѧ��Դ�����þ��зdz�������ǰ�����ش��������⣺
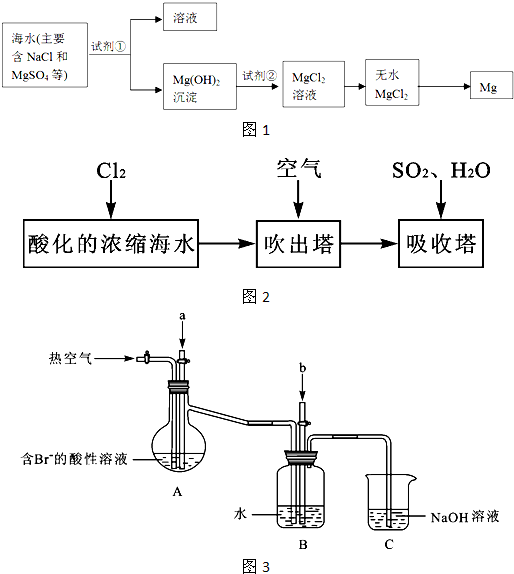
��1����ˮ������������������ͼ�Ǻ�ˮ����ԭ��ʾ��ͼ�������ص����豸�ṹ����������ˮ�����ã���Ҳ�������Ե�ȱ�ݣ�

1����Ϊ�����к�ˮ��������Ҫȱ����
����ʡij�غ���������������˽�һ�����ͺ�ˮ����������Ϊ�˷�����ˮ������ȱ�ݣ�����Ϊ�õ������һ������������
��2����ˮ������õ��ĵ�ˮӦ���м�����������ܵõ���ȫ��������������Ҫ���ˮ��ʹ�����ӽ�����֬��ˮ�е����ӽ��н����dz��õ�ˮ�����������۱�ϩ������һ�����ӽ�����֬����֪��ϩ���ƵĻ�ѧʽΪCH2=CH-COONa��д�����ɾ۱�ϩ���ƵĻ�ѧ���̣�
��3���Ӻ�ˮ�п��Ի�ȡʳ�Ρ�þ���ء��弰�仯����Ʒ��������������Ŀǰ�Ӻ�ˮ����ȡ��ij��÷����������������£�

��ʵ�����д����ᴿ�IJ�����
�ڷ�Ӧ�����ӷ���ʽ�ֱ�Ϊ
���ڷ�Ӧ�����ı����896m3SO2ʱ����ת��
��4���������������߷������������̵�������
��1����ˮ������������������ͼ�Ǻ�ˮ����ԭ��ʾ��ͼ�������ص����豸�ṹ����������ˮ�����ã���Ҳ�������Ե�ȱ�ݣ�

1����Ϊ�����к�ˮ��������Ҫȱ����
���Ĵ������������ɱ�̫��
���Ĵ������������ɱ�̫��
������ʡij�غ���������������˽�һ�����ͺ�ˮ����������Ϊ�˷�����ˮ������ȱ�ݣ�����Ϊ�õ������һ������������
���÷��ܻ�̫����
���÷��ܻ�̫����
����2����ˮ������õ��ĵ�ˮӦ���м�����������ܵõ���ȫ��������������Ҫ���ˮ��ʹ�����ӽ�����֬��ˮ�е����ӽ��н����dz��õ�ˮ�����������۱�ϩ������һ�����ӽ�����֬����֪��ϩ���ƵĻ�ѧʽΪCH2=CH-COONa��д�����ɾ۱�ϩ���ƵĻ�ѧ���̣�
nCH2=CH-COONa

| ���� |

nCH2=CH-COONa

��| ���� |

��3���Ӻ�ˮ�п��Ի�ȡʳ�Ρ�þ���ء��弰�仯����Ʒ��������������Ŀǰ�Ӻ�ˮ����ȡ��ij��÷����������������£�

��ʵ�����д����ᴿ�IJ�����
�ܽ⣬���ˣ������ᾧ
�ܽ⣬���ˣ������ᾧ
���ڷ�Ӧ���еõ�����Һ��ͨ�������ˮ����������������������
����������
���ڷ�Ӧ�����ӷ���ʽ�ֱ�Ϊ
2Cl-+2H2O
2OH-+Cl2��+H2����Cl2+2Br-=Br2+2Cl-��SO2+Br2+2H2O=4H++2Br-+SO42-
| ||
2Cl-+2H2O
2OH-+Cl2��+H2����Cl2+2Br-=Br2+2Cl-��SO2+Br2+2H2O=4H++2Br-+SO42-
��
| ||
���ڷ�Ӧ�����ı����896m3SO2ʱ����ת��
80000
80000
mol���ӣ���4���������������߷������������̵�������
�����嵥��
�����嵥��
����������1���ٴӳɱ��ĽǶȿ��ǣ�
����ij�غ�������̫���ܡ����ܣ����Խ���Щ����ת��Ϊ���ܣ�
��2�����ݼӾ۷�Ӧ��д��ѧ����ʽ��
��3���ٸ��ݴ��εijɷ֣�ѡ��ǡ���ķ������������ӷ���
�ڸ��ݷ�Ӧд����ѧ����ʽ��
�۸���һ��SO2ת�Ƶĵ�����Ŀ�Լ�SO2�����ʵ�����
��4�����������������Ҳ���壬�������ʵĸ�����
����ij�غ�������̫���ܡ����ܣ����Խ���Щ����ת��Ϊ���ܣ�
��2�����ݼӾ۷�Ӧ��д��ѧ����ʽ��
��3���ٸ��ݴ��εijɷ֣�ѡ��ǡ���ķ������������ӷ���
�ڸ��ݷ�Ӧд����ѧ����ʽ��
�۸���һ��SO2ת�Ƶĵ�����Ŀ�Լ�SO2�����ʵ�����
��4�����������������Ҳ���壬�������ʵĸ�����
����⣺��1�����������к�ˮ������Ҫ���Ͻ��м��ȣ��ɱ��ϴ� �ڿ���������ʽ�����������ã���̫���ܡ����ܣ�
��2����CH2=CH-COONa���ɾ۱�ϩ�����ǼӾ۷�Ӧ��nCH2=CH-COONa

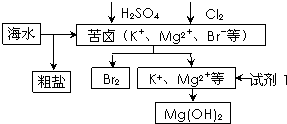
��3���ٴ��ε�������Ca2+��Mg2+��SO42- ����ɰ���ӹ�������������ȥ��������ټ������̼���Ƴ�ȥ��þ���ӹ����ټ�������������ᾧ�õ��ϴ������Ȼ��ƾ��壬����ˮ�֣�ԭ������ˮ���ξͻ�ᾧ�����������ӷ�����Һ��ͨ�������ˮ��������ʹ��ӷ�������
�ڷ�Ӧ��Ϊ��ⱥ��ʳ��ˮ��2NaCl+2H2O
2NaOH+Cl2��+H2��
��Ӧ��Ϊ�������������ӣ�Cl2+2Br-=Br2+2Cl-
��Ӧ��ΪBr2����SO2��SO2+Br2+2H2O=2HBr+H2SO4
��SO2�����ʵ���
=40000mol��SO2��S��+4�۱�Ϊ+6�ۣ�һ��SO2ת��2�����ӣ��ʹ�ת��80000mol���ӣ�
��4�������������Ҳ���壬������һ��Ϊ��ĸ�����
�ʴ�Ϊ����1�������Ĵ������������ɱ�̫�� �����÷��ܻ�̫��
��2��nCH2=CH-COONa

��3�����ܽ⣬���ˣ������ᾧ������������
��2Cl-+2H2O
2OH-+Cl2��+H2����Cl2+2Br-=Br2+2Cl-��SO2+Br2+2H2O=4H++2Br-+SO42-
��80000
��4�������嵥��
��2����CH2=CH-COONa���ɾ۱�ϩ�����ǼӾ۷�Ӧ��nCH2=CH-COONa
| ���� |

��3���ٴ��ε�������Ca2+��Mg2+��SO42- ����ɰ���ӹ�������������ȥ��������ټ������̼���Ƴ�ȥ��þ���ӹ����ټ�������������ᾧ�õ��ϴ������Ȼ��ƾ��壬����ˮ�֣�ԭ������ˮ���ξͻ�ᾧ�����������ӷ�����Һ��ͨ�������ˮ��������ʹ��ӷ�������
�ڷ�Ӧ��Ϊ��ⱥ��ʳ��ˮ��2NaCl+2H2O
| ||
��Ӧ��Ϊ�������������ӣ�Cl2+2Br-=Br2+2Cl-
��Ӧ��ΪBr2����SO2��SO2+Br2+2H2O=2HBr+H2SO4
��SO2�����ʵ���
| 896000L |
| 22.4l/mol |
��4�������������Ҳ���壬������һ��Ϊ��ĸ�����
�ʴ�Ϊ����1�������Ĵ������������ɱ�̫�� �����÷��ܻ�̫��
��2��nCH2=CH-COONa
| ���� |

��3�����ܽ⣬���ˣ������ᾧ������������
��2Cl-+2H2O
| ||
��80000
��4�������嵥��
���������⺣ˮˮ��Դ�����úͺ�ˮ��ѧ��Դ������Ϊ�����������֪ʶ��϶࣬������ѧ������֪ʶ���������������

��ϰ��ϵ�д�
�����Ŀ
����Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵��������ǣ�������

| A������BaCl2��Һ��ȥ�����е�SO42- | B���ӿ�±����ȡBr2�ķ�Ӧ�����ӷ���ʽΪ��2Br-+Cl2�T2Cl-+Br2 | C���Լ�1����ѡ��ʯ���� | D����ҵ�ϣ��������MgOұ������þ |
 ��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ�������
��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ�������