��Ŀ����
1��ʵ�������ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ��������250mL0.1mol/L��������Һ����ղ���ش��������⣺��1������250mL0.1mol/L��������Һ��������б���
| Ӧ��ȡŨ�������/mL | Ӧѡ������ƿ�Ĺ�� | ������ƿ��С�ձ�������������ͷ�ι����Ҫ�������������� |
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��250mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ�����2-3cm��
��3������A�У���ϴ��Һ����������ƿ����Ŀ���DZ�֤����ȫ��ת������ƿ��
��4�������������������������ҺŨ�ȵ�Ӱ���ǣ���ƫ��ƫС�����䣩��û�н���
A����ʱŨ��ƫС��������ˮʱ���������˿̶���ʱŨ��ƫС������ʱ����ʱŨ��ƫ��_��
���� ��1������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�������Һϡ���������ʵ����ʵ������������ҪŨ������������������һ�����ʵ���Ũ����Һ�IJ�������ѡ����Ҫ��������
��2����������һ�����ʵ���Ũ����Һ�IJ�����������
��3���ձ��Ͳ������϶�մ�����ᣬϴ��Һ�к������������ʣ�Ϊ�������ʵ����ļ��٣�Ӧ��ϴ��Һȫ��ת�Ƶ�����ƿ�У�
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1���ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ�������ʵ���Ũ��C=$\frac{1000��1.18��36.5%}{36.5}$=11.8mol/L������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�V��11.8mol/L=0.250L��0.1mol/L�����V=0.0021L����2.1mL��
����250mL������Ӧѡ��250mL����ƿ��
����һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���õ��������У�5mL��Ͳ�����������ձ���250mL����ƿ����ͷ�ιܣ���ȱ�ٵ�����Ϊ��5mL��Ͳ��
�ʴ�Ϊ��
| Ӧ��ȡŨ�������/mL | Ӧѡ������ƿ�Ĺ�� | ������ƿ��С�ձ�������������ͷ�ι����Ҫ�������������� |
| 2.1 | 250ml | 5ml��Ͳ |
�ʴ�Ϊ��B��C��A��F��E��D��
��3���ձ��Ͳ������϶�մ�����ᣬϴ��Һ�к������������ʣ�Ϊ�������ʵ����ļ��٣�Ӧ��ϴ��Һȫ��ת�Ƶ�����ƿ�У�
�ʴ�Ϊ����֤����ȫ��ת������ƿ��
��4��û�н���A����ʱ�����²�������ûת�Ƶ�����ƿ�����ʵ����ʵ���ƫС����ҺŨ��ƫС��
������ˮʱ���������˿̶���ʱŨ�ȣ�������Һ���ƫ����ҺŨ��ƫС��
����ʱ����ʱŨ�ȣ�������Һ���ƫС����ҺŨ��ƫ��
�ʴ�Ϊ��ƫС��ƫС��ƫ��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ��������ǽ���ؼ���ע���������ķ����ͼ��ɣ�

| A�� | ��֪�����ۻ���Ϊ6.0kJ/mol�������������Ϊ20kJ/mol������1mol������2mol��������ۻ�����ȫ�����ƻ���������������ֻ���ƻ�����30%����� | |
| B�� | һ���¶��£�������Һ���ʵ���Ũ��ΪC�������Ϊ����K��=$\frac{��Ca��^{2}}{C��1-a��}$������������CH3COONa���壬�����ƽ�������ƶ�������С��K������ | |
| C�� | ʵ���û����飨l��������ϩ��l���ͱ���l���ı�ȼ���ȷֱ�Ϊ-3916kJ/mol��-3747 kJ/mol��-3265 kJ/mol������֤���ڱ������в����ڶ�����̼̼˫�� | |
| D�� | �����к��ȷ�Ӧ�У���������¶ȼ��ϵ�����ˮ��ϴ������ô����cm��t����õ���Q�����ƫС |
 ʵ������50mL 0.50 mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50 mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
��1��������ͭ˿��������滷�β����������������Cu���ȿ죬������ʧ��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ������װ�õı���Ч���������ձ��粻��Ӳֽ�壬��õ��к�����ֵ��ƫС���ƫ����ƫС������Ӱ�족��������ճ�����ʵ�ʸ�ʵ���ڱ��±��У����ò�Ʒ��Ч�����ã�
��3�������ϱ����������ݽ��м��㣬���ʵ���õ��к��ȡ�H=-56.8kJ•mol-1[�����NaOH��Һ���ܶȰ�1g•cm-3���㣬��Ӧ������Һ�ı����ݣ�c����4.18J•��g•�棩-1����]��
��4������0.5mol/L��������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��ƫ��_���ƫ����ƫС���������䡱���������60mL0.5mol/L��������50mL 0.55mol•L-1 ��NaOH ��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ����������к�����ȣ����ȡ�����ȡ�����
��5����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����ABDF��
A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C������ʵ��ĵ������½ϸ�
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ
E������ȡ����ʱ���Ӽ���
F�����ձ��ĸǰ��м�С��̫��
| A�� | Ŀǰ�ӵ�ʳ����Ҫ���ӵ���KIO3 | |
| B�� | ��±�������ƶ��� | |
| C�� | ����������ˮ��ɱ�������� | |
| D�� | �ڿ��������ձ���SO2�������ڿ�����Ⱦָ�� |
| A�� | ����һ�����ʵ�����Ũ�ȵ���Һʱ������ʱ���ӿ̶��ᵼ��������ҺŨ��ƫ�� | |
| B�� | ��������ƽ��ȡ25.20g NaCl | |
| C�� | ��100mL ��Ͳ��ȡ5.2mL ���� | |
| D�� | ��Ũ��������һ�����ʵ���Ũ�ȵ�ϡ���ᣬ����ȡ��Ũ���ᵹ������ƿ�У���ˮϡ�͵��̶����� |
| A�� | �������� | B�� | �Ӵ���� | C�� | �����Ũ�� | D�� | �¶� |
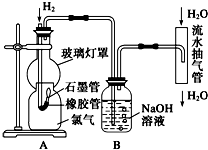 ijУ��ѧ��Ȥʵ��С������ˮ�����ܶԽ̲���������������ȼ�յ�ʵ������˸Ľ�������Ч�ط���Cl2��HCl�Ի�������Ⱦ����ʵ��װ����ͼ��ʾ��
ijУ��ѧ��Ȥʵ��С������ˮ�����ܶԽ̲���������������ȼ�յ�ʵ������˸Ľ�������Ч�ط���Cl2��HCl�Ի�������Ⱦ����ʵ��װ����ͼ��ʾ��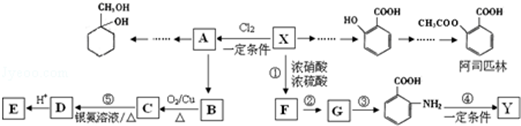

 ���������ױ�������
���������ױ�������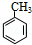 ����˴Ź�������ͼ��4�����շ壻
����˴Ź�������ͼ��4�����շ壻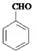 ��
��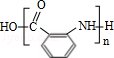 ��
��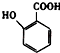 �ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯���ﹲ��6�֣�
�ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯���ﹲ��6�֣�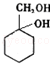 �����̣�A$��_{Na/��}^{H_{2}}$�ס���$\stackrel{Br_{2}/CCl_{4}}{��}$
�����̣�A$��_{Na/��}^{H_{2}}$�ס���$\stackrel{Br_{2}/CCl_{4}}{��}$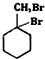 $��_{��}^{NaOH��aq��}$
$��_{��}^{NaOH��aq��}$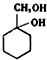
 ��
��