��Ŀ����
12�� ʵ������50mL 0.50 mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50 mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
��1��������ͭ˿��������滷�β����������������Cu���ȿ죬������ʧ��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ������װ�õı���Ч���������ձ��粻��Ӳֽ�壬��õ��к�����ֵ��ƫС���ƫ����ƫС������Ӱ�족��������ճ�����ʵ�ʸ�ʵ���ڱ��±��У����ò�Ʒ��Ч�����ã�
��3�������ϱ����������ݽ��м��㣬���ʵ���õ��к��ȡ�H=-56.8kJ•mol-1[�����NaOH��Һ���ܶȰ�1g•cm-3���㣬��Ӧ������Һ�ı����ݣ�c����4.18J•��g•�棩-1����]��
��4������0.5mol/L��������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��ƫ��_���ƫ����ƫС���������䡱���������60mL0.5mol/L��������50mL 0.55mol•L-1 ��NaOH ��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ����������к�����ȣ����ȡ�����ȡ�����
��5����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����ABDF��
A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C������ʵ��ĵ������½ϸ�
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ
E������ȡ����ʱ���Ӽ���
F�����ձ��ĸǰ��м�С��̫��
���� ��1������ͭ���ȿ죬������ʧ�ࣻ
��2���������кͷ�Ӧ�У�����ȷ��������ɢʧ�����������ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ���ճ����������Ǿ����õ����±����ڱ��±��н���ʵ�鱣��Ч������ã�
��3���ȸ��ݱ��вⶨ���ݼ�������Һ��Ӧǰ���ƽ���¶Ȳ�ٸ���Q=m•c•��T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��4���������ƹ�������ˮ���ų���������Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5������ʵ��ɹ��Ĺؼ��DZ��£����װ��������ɢʧ����ᵼ�½��ƫ�ͣ�����ʵ�����õ����Լ��Լ�ʵ�����֪ʶ���жϣ�
��� �⣺��1�����ܽ����β����������Ϊͭ˿���������Ϊͭ˿��������ȵ������壻
�ʴ�Ϊ��Cu���ȿ죬������ʧ��
��2�����кͷ�Ӧ�У�����ȷ��������ɢʧ��Ӧ���װ�õı���Ч�������ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С�����ճ�����ʵ�ʸ�ʵ���ڱ��±���Ч�����ã�
�ʴ�Ϊ�����װ�õı���Ч����ƫС�����±���
��3����1��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.25�棬��Ӧǰ���¶Ȳ�Ϊ��3.45�棻
��2��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.40�棬��Ӧǰ���¶Ȳ�Ϊ��3.40�棻
��3��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ21.55�棬��Ӧǰ���¶Ȳ�Ϊ��3.35�棻
�����¶Ȳ��ƽ��ֵΪ3.40�棬
50mL 0.50 mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ������m=100mL��1g/mL=100g��c=4.18J/��g•�棩�����빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g•�棩��100g��3.40��=1421.2J=1.4212KJ��������0.025mol��ˮ�ų�����1.4212KJ����������1mol��ˮ�ų�����Ϊ$\frac{1.4212kJ��1mol}{0.025mol}$=56.8kJ������ʵ���õ��к��ȡ�H=-56.8kJ/mol��
�ʴ�Ϊ��-56.8kJ/mol��
��4���������ƹ�������ˮ���ų�����������ʵ���в�õġ��к��ȡ���ֵ��ƫ��Ӧ�ų����������������Լ�������Ķ����йأ�����60mL0.5mol/L��������50mL 0.55mol•L-1 ��NaOH ��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ����к�����ָϡǿ����ϡǿ����кͷ�Ӧ����1molH2O�ų��������������������أ�����к�����ֵ��ȣ�
�ʴ�Ϊ��ƫ����ȣ���ȣ�
��5��A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С������ʵ�����к��ȵ���ֵƫС����A��ȷ��
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ����ᵼ��һ����������ɢʧ������ʵ�����к��ȵ���ֵƫС����B��ȷ��
C������ʵ������ºͷ�Ӧ�ȵ�����֮���أ���C����
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ�����ڰ�ˮ������������Ҫ���ȣ�����ʵ�����к��ȵ���ֵƫС����D��ȷ��
E������ȡ����ʱ���Ӽ�������ʹ��ʵ����ȡ���������Ҫ�������������������Ա�֤��ȫ��Ӧ������ʵ�����к��ȵ���ֵƫ�ߣ���E����
F�����ձ��ĸǰ��м�С��̫�ᵼ��һ��������ɢʧ������ʵ�����к��ȵ���ֵƫС����F��ȷ��
��ѡ��ABDF��
���� ���⿼�鷴Ӧ�ȵIJⶨ����㣬��Ŀ�ѶȲ���ע�������к��ȵĸ����Լ��ⶨ��Ӧ�ȵ��������⣮

| A�� | $\frac{c��O{H}^{-}��}{c��N{H}_{3}��{H}_{2}O��}$ | B�� | $\frac{c��N{H}_{3}•{H}_{2}O��}{c��O{H}^{-}��}$ | C�� | c��H+����c��OH-���ij˻� | D�� | OH-�����ʵ��� |
| A�� | ����������Һ�У�Cu2+��Fe3+��NO3-��Cl- | |
| B�� | ʹ���ȱ��ɫ����Һ��Fe2+��K+��NO3-��SO42- | |
| C�� | ���д���ClO-����Һ�У�K+��OH-��I-��SO32- | |
| D�� | ��ˮ���������c��H+��=10-12mol•L-1����Һ��NH4+��SO42-��HCO3-��Cl- |
��TiO2+2C+2Cl2 $\frac{\underline{\;����\;}}{\;}$ TiCl4+2CO ��TiCl4+2Mg $\frac{\underline{\;����\;}}{\;}$ 2MgCl2+Ti
������������ȷ���ǣ�������
| A�� | �ɷ�Ӧ�ٿ�֪��Cl2����������TiCl4���������� | |
| B�� | �ɷ�Ӧ�ٿ�֪������CO�ڸ����°�TiO2��ԭ��Ti | |
| C�� | �ɷ�Ӧ�ڿ�֪������24 g Mg�μӷ�Ӧ���Ϳ�����1 mol Ti | |
| D�� | �ɷ�Ӧ�ڿ�֪������Mg�Ļ�ԭ�ԱȽ���Ti�Ļ�ԭ��ǿ |
| A�� | �����ȣ��ܶ���ȵ�CO��C2H4 | B�� | �¶���ͬ�������ͬ��O2��N2 | ||
| C�� | ������ȣ��ܶȲ��ȵ�N2��CO | D�� | ѹǿ��ͬ�������ͬ��N2��O2 |
��1������250mL0.1mol/L��������Һ��������б���
| Ӧ��ȡŨ�������/mL | Ӧѡ������ƿ�Ĺ�� | ������ƿ��С�ձ�������������ͷ�ι����Ҫ�������������� |
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��250mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ�����2-3cm��
��3������A�У���ϴ��Һ����������ƿ����Ŀ���DZ�֤����ȫ��ת������ƿ��
��4�������������������������ҺŨ�ȵ�Ӱ���ǣ���ƫ��ƫС�����䣩��û�н���
A����ʱŨ��ƫС��������ˮʱ���������˿̶���ʱŨ��ƫС������ʱ����ʱŨ��ƫ��_��
| A�� | ̼������Һ | B�� | �Ȼ�����Һ | ||
| C�� | ���Ը��������Һ | D�� | ��ˮ |
 ��ͼ��һ��ԭ���װ�ã��밴Ҫ����գ�
��ͼ��һ��ԭ���װ�ã��밴Ҫ����գ�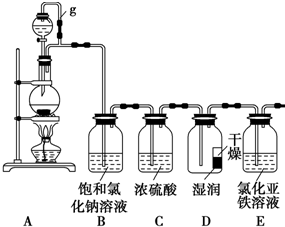 ij̽��С��Ϊ̽�����������ʣ����������ʵ��װ�ã���ش��������⣺
ij̽��С��Ϊ̽�����������ʣ����������ʵ��װ�ã���ش��������⣺