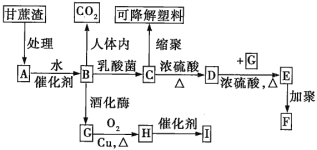
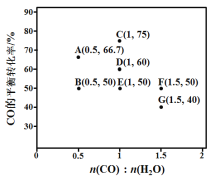
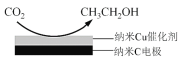
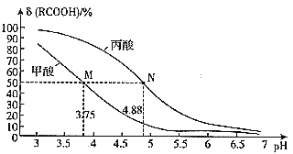
��Ŀ����
����Ŀ��ij����ֻ��C��H��O����Ԫ�أ�����ӵ����ģ����ͼ��ʾ�������й���12��ԭ��(ͼ��������֮������ߴ���������˫���Ȼ�ѧ��)��
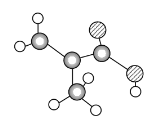
(1)�������к��������ŵĽṹ��ʽΪ________��
(2)���������У�������ʻ�Ϊͬ���칹�����________(�����)��
A��CH3CH2CH2COOH
B��OHCCH(CH3)CHO
C��CH3CH2CH===CHCOOH
D��CH2===CHCOOCH3
(3)�÷����й�ƽ���ԭ�������Ϊ________(��֪�Ȼ����ĸ�ԭ�ӿ��Թ�ƽ��)��
���𰸡���COOH BD 10
��������
�������ʳɼ��ص��֪������4����������C����һ���жϳ�С����ΪH����һ��ΪO��
(1)���ݷ���ģ�Ϳ�֪�������ʵĽṹ��ʽΪCH2===C(CH3)COOH������������Ϊ-COOH����Ϊ��-COOH��
(2) A. CH3CH2CH2COOH��CH2===C(CH3)COOH����ʽ��ͬ��A����
B. OHCCH(CH3)CHO��CH2===C(CH3)COOH����ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬B��ȷ��
C. CH3CH2CH===CHCOOH��CH2===C(CH3)COOH����ʽ��ͬ��C����
D. CH2===CHCOOCH3��CH2===C(CH3)COOH����ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬D��ȷ����Ϊ��BD��
(3)̼̼˫����ƽ��ṹ���Ȼ����ĸ�ԭ�ӿ��Թ�ƽ�棬��ͼ��ʾ���÷����й�ƽ���ԭ�������Ϊ10��(���С�����)��
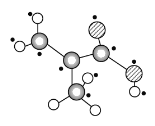
����10��

����Ŀ��������ѧ֪ʶ�ش��������⣺
��1��������ʵ���ã�5g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ��___��
��2���������������ȼ�յ��Ȼ�ѧ����ʽΪSi(s)+O2(g)=SiO2(s) ��H=-989.2kJ��mol-1���йؼ����������±���
��ѧ�� | Si��O | O=O | Si��Si |
����/kJ��mol-1 | x | 498.8 | 176 |
��֪1molSi�к�2molSi��Si����1molSiO2�к�4molSi��O������x��ֵΪ__��
��3��AgNO3��ˮ��Һ��___�����������������������������ԣ�ʵ����������AgNO3����Һʱ������AgNO3���������ڽ�Ũ�������У�Ȼ����������ˮϡ�͵������Ũ�ȣ�Ŀ����___��
��4����ϡ�ʹ���Ĺ����У�����ʼ�ձ����������Ƶ�������______��
A.c(H+) B.H+���� C.CH3COOH������ D.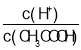
��5��ij�¶�(t��)ʱ��ˮ�����ӻ�ΪKw=1��10-13���������¶���pH=11�Ŀ�������ҺaL��pH=1��ϡ����bL���(���Ϻ���Һ�����С�仯���Բ���)�������û����Һ��pH=2����a��b=__��
��6����֪Cr(OH)3����Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s)![]() Cr3+(aq)+3OH-(aq)�������£�Cr(OH)3���ܶȻ�Ksp=10-32��Ҫʹc(Cr3+)����10-5mol��L-1����Һ��pHӦ���ٵ���__��
Cr3+(aq)+3OH-(aq)�������£�Cr(OH)3���ܶȻ�Ksp=10-32��Ҫʹc(Cr3+)����10-5mol��L-1����Һ��pHӦ���ٵ���__��