��Ŀ����
��1��������Bunsen���Ȼ�ѧѭ������SO2����������������Ӧ��ɣ�
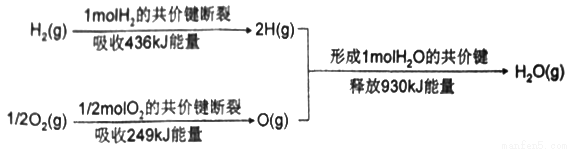
2H2(g)+O2(g)=2H2O(l) ��H1=-572kJ��mol-1
2HI(g)=H2(g)+I2(g) ��H2=+10kJ��mol-1
2H2SO4(l)=2SO2(g)+2H2O(l)+O2(g) ��H3=+462kJ��mol-1
���
SO2(g)+I2(g)+2H2O(I)=2HI (g)+H2SO4(I) ��H=_______ kJ��mol-1
��2������п���շ�������ZnO����Һ�����������з��ѭ�������pH������Ч�ʦ���ʱ��t�ı仯��ͼ1��ʾ����Һ�в�������PH�Ĺ�ϵ��ͼ2��ʾ��


��Ϊ���SO2������Ч�ʦǣ��ɲ�ȡ�Ĵ�ʩ�У���������Һ��ZnO������________________��
��ͼ1�е�pH-t����ab�η�������Ҫ��ѧ����ʽΪ___________________
��pH=7ʱ����Һ�� =________
=________
��3����ͼ3��ʾ�����ö��Ե���⺬SO2����������S��H2SO4����ʵ�ַ������á�

�������ĵ缫��ӦʽΪ_____________��
��ÿ������19.2g SO2�������������ϻ���S��H2SO4�����ʵ����ֱ�Ϊ______��________��
��ϰ��ϵ�д�
�����Ŀ


 H2(g)+I2(g)����֪H2(g)��I2(g)����ʼ���ʵ�����Ϊ0��HI(g)�����ʵ���(mol)�淴Ӧʱ��(min)�ı仯������±���ʾ�������¶ȵĵ�λΪ���϶�(��)��
H2(g)+I2(g)����֪H2(g)��I2(g)����ʼ���ʵ�����Ϊ0��HI(g)�����ʵ���(mol)�淴Ӧʱ��(min)�ı仯������±���ʾ�������¶ȵĵ�λΪ���϶�(��)��

 Mn2++2Cl-+Cl2��+2H2O
Mn2++2Cl-+Cl2��+2H2O