��Ŀ����
����Ŀ��������ͼ��ʾ��ת����ϵ��ˮ�Ͳ��ֲ�������ȥ��
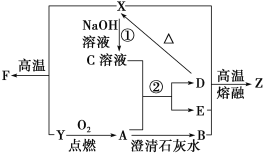
��֪����X��Z����������������ˮ�ļ�Ӳ���壬����Z�̶��۵㣬���ִ���������ȱ�ٵ�װ�κͲɹ���ϣ�����ɫ����A����������ЧӦ����Ҫ���壻
��B��D��Ϊ������ˮ�İ�ɫ���壻�ܸߴ��ȵ�F��ʹ����㷺�İ뵼����ϡ�
�ݴ˻ش��������⣺
��1��Z��������________������ʦ��Z�����ʴ����ͼ�����õ��Լ�Ϊ________(������)��
��2����X��Y��F�Ļ�ѧ����ʽΪ________________________���˷�Ӧ��Y��________��(���������ԭ��)��
��3��ת���ٵĻ�ѧ����ʽΪ________________________��ת����(A����)�����ӷ���ʽΪ______________________��
���𰸡����� ����� SiO2+2CO![]() 2CO2+Si ��ԭ SiO2��2NaOH===Na2SiO3��H2O SiO
2CO2+Si ��ԭ SiO2��2NaOH===Na2SiO3��H2O SiO![]() ��CO2��H2O===H2SiO3����CO
��CO2��H2O===H2SiO3����CO![]()
��������
��XΪ������ˮ�ļ�Ӳ���壬Z�̶��۵㣬���ִ������в���ȱ�ٵ�װ�β��ϣ��ж�Ϊ������˵��XΪSiO2��
����ɫ����A����������ЧӦ����Ҫ����ΪCO2��
��B��D��Ϊ������ˮ�İ�ɫ���壬���̷�����֪BΪCaCO3����
�ܸߴ��ȵ�F���������оƬ��һ�ַǽ�������ΪSi��
������̷����жϿ�֪XΪSiO2��BΪCaCO3��CΪNa2SiO3��DΪH2SiO3��EΪNa2CO3��ZΪ������YΪCO��FΪSi��
�����ϣ�1��Z�̶��۵㣬���ִ������в���ȱ�ٵ�װ�β��ϣ��ж�Ϊ����������ʦ��Z�����ʴ����ͼ�����õ��Լ�Ϊ����ᡣ
��2����X��Y��F�Ĺ���ΪSiO2+2CO![]() 2CO2+Si��CO����ԭ����
2CO2+Si��CO����ԭ����
��3��ת���ٵĻ�ѧ����ʽΪSiO2��2NaOH===Na2SiO3��H2O ����ɫ����A����������ЧӦ����Ҫ���壬��AΪCO2��ת����(A����)�����ӷ���ʽΪSiO![]() ��CO2��H2O===H2SiO3����CO
��CO2��H2O===H2SiO3����CO![]() ��
��

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
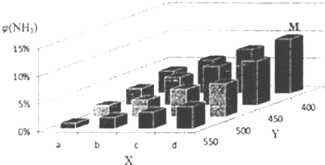
��У������Ԫͬ��ѵ��������ϵ�д�����Ŀ��1913�꣬�¹���ѧ�ҹ���ʵ���˺ϳɰ��Ĺ�ҵ�����������������������ʳΣ���Ļ�ѧ��ţ��ֽ�lmolN2��3molH2Ͷ��1L���ܱ���������һ�������£��������·�Ӧģ������ϳɰ��Ĺ�ҵ��������N2(g)+3H2(g) ![]() 2NH3(g)��H��0�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��NH3�����������(NH3)�仯������ͼ��ʾ��
2NH3(g)��H��0�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��NH3�����������(NH3)�仯������ͼ��ʾ��
�ش��������⣺
��1����֪����NH3(l)�TNH3(g)��H1����N2(g)+3H2(g) ![]() 2NH3(l)��H2����ӦN2(g)+3H2(g)
2NH3(l)��H2����ӦN2(g)+3H2(g) ![]() 2NH3(g)����H=_____________(�ú���H1����H2�Ĵ���ʽ��ʾ)��
2NH3(g)����H=_____________(�ú���H1����H2�Ĵ���ʽ��ʾ)��
��2���ϳɰ���ƽ�ⳣ������ʽΪ____________��ƽ��ʱ��M��NH3���������Ϊ10%����N2��ת����Ϊ____________(������λ��Ч����) ��
��3��X����a�����ֵ��b��____________(������������С��)����ͼ�У�Y���ʾ____________(�����¶�������ѹǿ��)���жϵ�������____________��
��4������1mol N2��3mol H2�ֱ�Ͷ����ʼ�ݻ�Ϊ1L���ܱ������У�ʵ��������ƽ��ʱ��������������ʾ��
������� | ʵ������ | ƽ��ʱ��Ӧ�е������仯 |
�� | ���º��� | ����Q1kJ |
�� | ���º�ѹ | ����Q2kJ |
�� | ���ݾ��� | ����Q3kJ |
�����ж���ȷ����____________��
A���ų�������Ql��Q2����Hl B��N2��ת���ʣ���
C��ƽ�ⳣ������ D����ƽ��ʱ�����������������
��5�������£���VmL amol/L��ϡ������Һ�еμӵ����bmol/L�İ�ˮ��ǡ��ʹ�����Һ�����ԣ���ʱ��Һ��c(NH4+)____________c(SO42-)(��������������������=��) ��
��6�����ð������һ�ֻ���ȼ�ϵ�أ�һ��ͨ�백������һ��ͨ�������������Dz���������(Y2O3)�������(ZrO2)���壬��������״̬���ܴ���O2-��д�������ĵ缫��Ӧʽ____________��