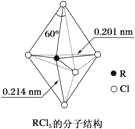
��Ŀ����
����Ŀ����(B)�������仯�����ڻ�ѧ������Ҫ�ĵ�λ����ش��������⣺
��.��1����B�Ļ�̬ԭ�ӵ����Ų�ʽ�гɵ���������ͬ�ĵڶ�����Ԫ�ػ���______����дԪ�ط��ţ���
��.����(H3BO3)�ǰ�ɫƬ״���壨��״�ṹ��ͼ1�����л���У�����ˮ���ܽ�Ⱥ�С������ʱ�ܽ������
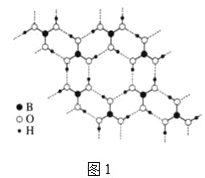
��2����������Է��»��������ۼ�������γɻ�״�ṹ��ÿ������Bԭ�ӵĻ���ƽ������Bԭ�ӵĸ���Ϊ______��������Bԭ�ӵ��ӻ�����Ϊ______��
��3������ʱ��������ܽ��������Ҫԭ����______��
��4��������һԪ���ᣬ�����ʽ���дΪB(OH)3����ˮ�е���ʱ��������ˮ�������OH-�Ķ������ԣ�д������ĵ��뷽��ʽ______��
��5��������(NH3BH3)��һ�����ʹ�����ϣ�������д�����λ������������ӵĽṹʽ��ṹ��ʽΪ______
������������
��.���⻯����һ�ֳ��õĻ�ԭ�����侧���ṹ��ͼ2��ʾ��
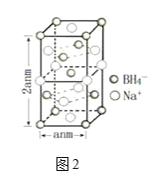
��6���þ�����Na+����λ��Ϊ______��
��7�������⻯�ƾ������µ��Ĵ���Na+��Li+ȡ�����õ��ľ���Ļ�ѧʽΪ______��
��8��LiAlH4Ҳ��һ������Ļ�ԭ�����ɽ�����ֱ�ӻ�ԭ�ɴ���CH3COOH![]() CH3CH2OH��CH3COOH�����м���2______����1������������������������������
CH3CH2OH��CH3COOH�������2______����1������������������������������
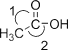
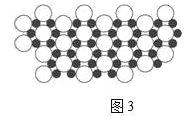
��.��9����þ������39Kʱ�ʳ����ԡ�����þ�����У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ���ͼ3�Ǹþ����۽ṹ����ͼ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬһƽ���ϡ�����þ�Ļ�ѧʽΪ______��
���𰸡�Li��F ![]() sp2 �����ƻ����������֮��������������������ˮ�γ�����ļ��� H3BO3+H2O
sp2 �����ƻ����������֮��������������������ˮ�γ�����ļ��� H3BO3+H2O![]() [B(OH)4]-+H+
[B(OH)4]-+H+ 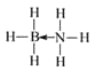 8 Na3Li(BH4)4 �� MgB2
8 Na3Li(BH4)4 �� MgB2
��������
��.��1��B�Ļ�̬ԭ�ӵ����Ų�ʽΪ 1s22s22p1����B�Ļ�̬ԭ�ӵ����Ų�ʽ�гɵ���������ͬ�ĵڶ�����Ԫ�ػ���Li��F������Ԫ�������ֻ��1�������ӡ��ʴ�Ϊ��Li��F��
��.��2����������Է��»��������ۼ�������γɻ�״�ṹ��ÿ��������2��Bԭ�ӣ�ÿ��B��3������̯��ÿ������Bԭ�ӵĻ���ƽ������Bԭ�ӵĸ���Ϊ2��![]() =
=![]() ������ͼ��֪��Bԭ���γ�3��B-O������û�йµ��Ӷԣ�Bԭ���ӻ������ĿΪ3��Bԭ�Ӳ�ȡsp2�ӻ���ʽ��
������ͼ��֪��Bԭ���γ�3��B-O������û�йµ��Ӷԣ�Bԭ���ӻ������ĿΪ3��Bԭ�Ӳ�ȡsp2�ӻ���ʽ��
�ʴ�Ϊ��![]() ��sp2 ��
��sp2 ��
��3������ʱ��������ܽ��������Ҫԭ���Ǽ����ƻ����������֮��������������������ˮ�γ�����ļ��ʡ�
�ʴ�Ϊ�������ƻ����������֮��������������������ˮ�γ�����ļ��ʣ�
��4��������һԪ���ᣬ�����ʽ���дΪB(OH)3����ˮ�е���ʱ��������ˮ�������OH-�Ķ������ԣ�����ĵ��뷽��ʽH3BO3+H2O![]() [B(OH)4]-+H+��
[B(OH)4]-+H+��
�ʴ�Ϊ��H3BO3+H2O![]() [B(OH)4]-+H+��
[B(OH)4]-+H+��
��5��������(NH3BH3)��һ�����ʹ�����ϣ�B�пչ����N�йµ��Ӷԣ�������д���![]() ��λ������������ӵĽṹʽ��ṹ��ʽΪ
��λ������������ӵĽṹʽ��ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��.��6�����ݾ����ṹ����������Na����Χ�Ⱦ��������BH4��������ΪNa������λ������Na������λ��Ϊ8���ʴ�Ϊ��8��
��7�������ж�������ռ![]() ����������ռ
����������ռ![]() ����������ռ
����������ռ![]() �������⻯�ƾ������µ��Ĵ��� Na���� Li��ȡ������BH4������Ϊ4��Na����ĿΪ3��Li����ĿΪ1�����Եõ��ľ���Ļ�ѧʽΪ��Na3Li(BH4)4 ���ʴ�Ϊ��Na3Li(BH4)4 ��
�������⻯�ƾ������µ��Ĵ��� Na���� Li��ȡ������BH4������Ϊ4��Na����ĿΪ3��Li����ĿΪ1�����Եõ��ľ���Ļ�ѧʽΪ��Na3Li(BH4)4 ���ʴ�Ϊ��Na3Li(BH4)4 ��
��8�� ��C=O˫������ԭ�Ӻ���2�Թ¶Ե��ӣ����ڹ¶Ե�����ɼ����Ӷ��ų������ڳɼ����Ӷ�֮���ų�������CH3COOH�����м���2������1��
��C=O˫������ԭ�Ӻ���2�Թ¶Ե��ӣ����ڹ¶Ե�����ɼ����Ӷ��ų������ڳɼ����Ӷ�֮���ų�������CH3COOH�����м���2������1��
�ʴ�Ϊ������
��.��9���þ����۽ṹ����ͼ�У�ÿ��Mgԭ����Χ��6��Bԭ�ӣ�ÿ��Bԭ��Ϊ3��Mgԭ�ӹ��ã�ƽ��һ��Mgԭ��ӵ�е�Bԭ��Ϊ![]() ��6=2��Mg����ԭ�Ӹ�����Ϊ1��2����þ�Ļ�ѧʽΪMgB2���ʴ�Ϊ��MgB2��
��6=2��Mg����ԭ�Ӹ�����Ϊ1��2����þ�Ļ�ѧʽΪMgB2���ʴ�Ϊ��MgB2��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�