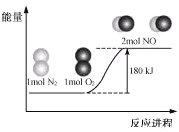
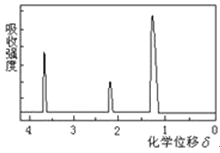
��Ŀ����
����Ŀ��B��C��N��Si�Ǽ��ֳ�������Ҫ�ǽ���Ԫ�أ����γɵĸ��ֻ���������Ȼ���й㷺���ڡ�
(1)��̬��ԭ�ӵĵ����Ų�ʽΪ________________��C��N��OԪ��ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________________��д��һ����CO32-��Ϊ�ȵ�����������ӣ�_________��
(2)BF3��һ������ˮ���γ���ͼ��ʾ����R

�پ���R�и���������������漰___________(����ĸ����)��
a.���Ӽ� b.���ۼ� c.��λ�� d.������ e.���»���
�ھ���R�������ӵĿռ乹��Ϊ________________________��
(3)�Ҷ���(H2NCH2CH2NH2)��CuCl2��Һ���γ�������(�ṹ��ͼ��ʾ)���Ҷ��������е�ԭ�ӵ��ӻ�����Ϊ________________��
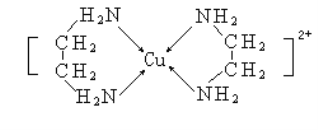
�Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵö࣬��ԭ����__________��
���𰸡�1s22s22p63s23p2 N��O��C NO3-(��SiO32-��) a��b��c �������� sp3 �Ҷ�������֮������γ���������װ�����֮�䲻���γ����
��������
(1)Siλ�����ڱ��е�3���ڵڢ�A�壬ͬ��������Ԫ������ԭ����������һ�����ܳ���������ƣ����ڢ�A��͵ڢ�A��Ԫ�ط������ȵ�������ָԭ��������ͬ���۵���������ͬ������
(2)�ٸ��ݾ���R�Ľṹ�������������Ӽ������ۼ�����λ����
�ھ���R��������ΪBF3(OH)-�������ӻ�������۷�����ռ乹�ͣ�
(3)�Ҷ�����N�γ�3�����ۼ�����������һ�Թµ��Ӷԣ��Ҷ��������װ��ķе�ߵö࣬�����Ҷ����γɷ��Ӽ������
(1)Siλ�����ڱ��е�3���ڵڢ�A�壬���̬��ԭ�ӵĵ����Ų�ʽΪ��1s22s22p63s23p2;
ͬ��������Ԫ������ԭ����������һ�����ܳ���������ƣ����ڢ�A��͵ڢ�A��Ԫ�ط�����C��N��OԪ��ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ��N��O��C��
�ȵ�������ָԭ��������ͬ���۵���������ͬ��������CO32-��Ϊ�ȵ�������������У�NO3-(��SiO32-)�ȣ�
(2)�ٸ��ݾ���R�Ľṹ�������������Ӽ������ۼ�����λ�����ʺ���ѡ����abc��
�ھ���R��������ΪBF3(OH)-��B�ļ۲����Ϊ2s22p1���������γ��ĸ����ۼ��������ӻ�������ۣ�ӦΪsp3�ӻ������ӵĿռ乹��Ϊ�������Σ�
(3)�Ҷ�����N�γ�3�����ۼ�����������һ�Թµ��Ӷԣ������ӻ�������ۣ���Nԭ�ӵ��ӻ���ʽΪsp3���Ҷ��������װ��ķе�ߵö࣬�����Ҷ����γɷ��Ӽ�����������˷���֮�����������ʹ����������Ҫ�����������

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�