ЬтФПФкШн
ЁОЬтФПЁПЂё.ЪЕбщЪввЊХфжЦ500 mL 0.2 mol/L NaOHШмвКЃЌЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉХфжЦЙ§ГЬжаВЛашвЊЪЙгУЕФЛЏбЇвЧЦїга________ЃЈЬюзжФИЃЉЁЃ
A ЩеБ B 500 mLШнСПЦП C ТЉЖЗ D НКЭЗЕЮЙм E ВЃСЇАє
ЃЈ2ЃЉгУЭаХЬЬьЦНГЦШЁЧтбѕЛЏФЦЃЌЦфжЪСПЮЊ________ gЁЃ
ЃЈ3ЃЉЯТСажївЊВйзїВНжшЕФе§ШЗЫГађЪЧ________ЃЈЬюађКХЃЉЁЃ
ЂйГЦШЁвЛЖЈжЪСПЕФЧтбѕЛЏФЦЃЌЗХШыЩеБжаЃЌгУЪЪСПеєСѓЫЎШмНтЃЛ
ЂкМгЫЎжСвКУцРыШнСПЦПЦПОБПЬЖШЯпЯТ1ЁЋ2 cmЪБЃЌИФгУНКЭЗЕЮЙмЕЮМгеєСѓЫЎжСАМвКУцгыПЬЖШЯпЯрЧаЃЛ
ЂлД§РфШДжСЪвЮТКѓЃЌНЋШмвКзЊвЦЕН500 mLШнСПЦПжаЃЛ
ЂмИЧКУЦПШћЃЌЗДИДЩЯЯТЕпЕЙЃЌвЁдШЃЛ
ЂнгУЩйСПеєСѓЫЎЯДЕгЩеБФкБкКЭВЃСЇАє2ЁЋ3ДЮЃЌЯДЕгвКзЊвЦЕНШнСПЦПжаЁЃ
ЃЈ4ЃЉШчЙћЪЕбщЙ§ГЬжаШБЩйВНжшЂнЃЌЛсЪЙХфжЦГіЕФNaOHШмвКХЈЖШ_______ЃЈЬюЁАЦЋИпЁЂЦЋЕЭЁБЛђЁАВЛБфЁБЃЉЁЃ
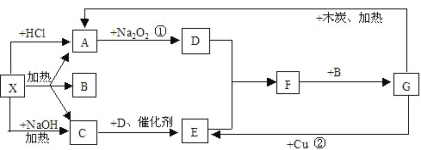
Ђђ.ШчЭМЪЕбщЪвФГХЈбЮЫсЪдМСЦПБъЧЉЩЯЕФгаЙиЪ§ОнЃЌЪдИљОнБъЧЉЩЯЕФгаЙиЪ§ОнЛиД№ЯТСаЮЪЬтЃК
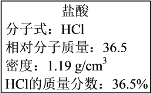
ЃЈ1ЃЉИУХЈбЮЫсжаHClЕФЮяжЪЕФСПХЈЖШЮЊ______ mol/LЁЃ
ЃЈ2ЃЉШЁгУШЮвтЬхЛ§ЕФИУбЮЫсШмвКЪБЃЌЯТСаЮяРэСПжаВЛЫцЫљШЁЬхЛ§ЕФЖрЩйЖјБфЛЏЕФЪЧ______ЁЃ
A ШмвКжаHClЕФЮяжЪЕФСПЁЁЁЁ B ШмвКЕФХЈЖШ
C ШмвКжаClЃЕФЪ§ФП D ШмвКЕФУмЖШ
ЃЈ3ЃЉФГбЇЩњгћгУЩЯЪіХЈбЮЫсКЭеєСѓЫЎХфжЦ500 mLЮяжЪЕФСПХЈЖШЮЊ0.400 mol/LЕФЯЁбЮЫсЁЃИУбЇЩњашвЊСПШЁ______mLЩЯЪіХЈбЮЫсНјааХфжЦЁЃ
Ђѓ.Яжга0.27KgжЪСПЗжЪ§ЮЊ10ЃЅЕФCuCl2ШмвК,дђШмвКжаCuCl2ЕФЮяжЪЕФСПЮЊ___________
ЁОД№АИЁПC 4.0g ЂйЂлЂнЂкЂм ЦЋЕЭ 11.9 BD 16.8 0.2
ЁОНтЮіЁП
Ђё.ЃЈ1ЃЉШмвКХфжЦЙ§ГЬжаашвЊЩеБШмНтЙЬЬхЃЌашвЊ500mLШнСПЦПХфжЦШмвКЃЌашвЊНКЭЗЕЮЙмЖЈШнЃЌашвЊВЃСЇАєНСАшКЭв§СїЃЌЫљвдУЛгагУЕНЕФЪЧТЉЖЗЃЌЙЪбЁCЃЛ
ЃЈ2ЃЉгУЭаХЬЬьЦНГЦШЁЧтбѕЛЏФЦЃЌЦфжЪСПЮЊm=cЁСVЁСM=0.2mol/LЁС0.5LЁС40g/mol=4.0gЃЌЙЪД№АИЮЊЃК4.0gЃЛ
ЃЈ3ЃЉЪЕбщВйзїЕФВНжшЃКМЦЫуЁЂГЦСПЁЂШмНтЁЂвЦвКЁЂЯДЕгвЦвКЁЂЖЈШнЁЂвЁдШЕШВйзїНјааХХађЃЌЫљвдЦфХХСаЫГађЮЊЃКЂйЂлЂнЂкЂмЃЌЙЪД№АИЮЊЃКЂйЂлЂнЂкЂмЃЛ
ЃЈ4ЃЉвђЯДЕгвКжаКЌгаШмжЪЃЌЮДНЋЯДЕгвКзЊШыШнСПЦПЃЌШмжЪЕФжЪСПМѕЩйЃЌХЈЖШЦЋЕЭЃЌЙЪД№АИЮЊЃКЦЋЕЭЃЛ
Ђђ.ЃЈ1ЃЉЩшбЮЫсЕФЬхЛ§ЮЊ1LЃЌдђШмжЪЕФжЪСПЮЊ1000mLЁС1.19g cm-3ЁС36.5%ЃЌШмжЪЕФЮяжЪЕФСПЮЊ![]() =11.9molЃЌЫљвдШмжЪЕФЮяжЪЕФСПХЈЖШЮЊ
=11.9molЃЌЫљвдШмжЪЕФЮяжЪЕФСПХЈЖШЮЊ![]() =11.9mol/LЃЌЙЪД№АИЮЊЃК11.9ЃЛ
=11.9mol/LЃЌЙЪД№АИЮЊЃК11.9ЃЛ
ЃЈ2ЃЉA.n=cVЃЌЫљвдгыШмвКЬхЛ§гаЙиЃЌЙЪAДэЮѓЃЛB.ШмвКЕФХЈЖШЪЧОљвЛЮШЖЈЕФЃЌгыЫљШЁШмвКЕФЬхЛ§ЮоЙиЃЌЙЪBе§ШЗЃЛC.N=nNA=cVNAЃЌЫљвдгыШмвКЬхЛ§гаЙиЃЌЙЪCДэЮѓЃЛ D.ШмвКЕФУмЖШЪЧОљвЛЕФЃЌЫљвдгыЫљШЁШмвКЕФЬхЛ§ЮоЙиЃЌЙЪDе§ШЗЃЛЙЪбЁЃКBDЃЛ
ЃЈ3ЃЉИљОнШмвКЯЁЪЭЧАКѓШмжЪЕФСПВЛБфПЩжЊЃЌV(ХЈбЮЫс)ЁС11.9=0.400molЁЄLЃ1ЁС0.5LЃЌV(ХЈбЮЫс)= 0.0168L=16.8mLЃЛзлЩЯЫљЪіЃЌБОЬтД№АИЪЧЃК16.8ЃЛ
Ђѓ. ИљОнжЪСПЗжЪ§ЃЌПЩвдЧѓЕУТШЛЏЭШмвКжаКЌгаТШЛЏЭЕФжЪСПЮЊЃК0.27KgЁС0.1=0.027KgЃЌМДЮЊ27gТШЛЏЭШмвКЃЌЧѓЕУЦфЮяжЪЕФСПЮЊ![]() =0.2molЃЛ
=0.2molЃЛ

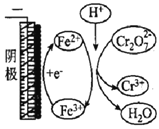
ЁОЬтФПЁПгУЕчНтЗЈДІРэКЌCr2O72ЃЕФЗЯЫЎЃЌЬНОПВЛЭЌвђЫиЖдКЌCr2O72ЃЗЯЫЎДІРэЕФгАЯьЃЌНсЙћШчБэЫљЪО(Cr2O72ЃЕФЦ№ЪМХЈЖШЁЂЬхЛ§ЁЂЕчбЙЁЂЕчНтЪБМфОљЯрЭЌ)ЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ
ЪЕбщ | i | ii | iii | iv |
|
ЪЧЗёМгШыFe2(SO4)3 | Зё | Зё | МгШы30g | Зё | |
ЪЧЗёМгШыH2SO4 | Зё | МгШы1mL | МгШы1mL | МгШы1mL | |
вѕМЋДхСЯ | ЪЏФЋ | ЪЏФЋ | ЪЏФЋ | ЪЏФЋ | |
бєМЋВФСЯ | ЪЏФЋ | ЪЏФЋ | ЪЏФЋ | Ьњ | |
Cr2O72ЃЕФШЅГ§ТЪ | 0.092% | 12.7% | 20.8% | 57.3% | ЪЕбщiiiжаFe3+ШЅГ§Cr2O72ЃЕФЛњРэ |
A. ЪЕбщЂЂгыЪЕбщiЖдБШЃЌЦфЫћЬѕМўВЛБфЃЌдіМгc(H+)гаРћгкCr2O72ЃЕФШЅГ§
B. ЪЕбщЂЃгыЪЕбщЂЂЖдБШЃЌЦфЫћЬѕМўВЛБфЃЌдіМгc(Fe3+)гаРћгкCr2O72ЃЕФШЅГ§
C. ЪЕбщЂЄжаFe2+бЛЗРћгУЬсИпСЫCr2O72ЃЕФШЅГ§ТЪ
D. ШєЪЕбщЂЄжаШЅГ§0.01 mol Cr2O72ЃЃЌЩњГЩЕФбєРызгШЋВПзЊЛЏГЩГСЕэЃЌдђГСЕэЕФжЪСПЪЧ2.06g