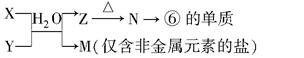��Ŀ����
����������SnSO4�������Ȼ�����SnCl4��������ӡȾ�͵�ƹ�ҵ��
��1��ij�о�С�����SnSO4�Ʊ�·�����£�
��֪�����������£�����ˮ��Һ����Sn2����Sn4��������Ҫ������ʽ��Sn2���ױ�������SnCl2����ˮ�⡣
��SnCl2���ܺ����Sn�۵������� ��
�ڲ�������õ��IJ����������ձ���� �����������Ҫϴ�ӹ���SnO�к��е����ʣ�����SnO�е�Cl���Ƿ�ϴ�Ӹɾ��IJ���Ϊ ��
�۲�����漰���IJ����У�a.���� b.ϴ�� c.����Ũ�� d.��ȴ�ᾧ e.���¸��������ȷ�IJ���˳��Ϊ ��
��2��ʵ������������װ�ã������ڵĽ���������﴿����������ȡ��ˮSnCl4��SnCl4�۵㣭33�棬�е�114.1�棬����ʪ��������ˮ�⣩���˷�Ӧ���̷ų��������ȡ�
��װ��C��Ӧ�����Լ�Ϊ___________������E������Ϊ____________________��
�ڷ�Ӧ��ʼ����SnCl4ʱ��������Ϩ��___������ĸ��ţ����ľƾ��ƣ�������________��
�۸�ʵ��װ������д���ȱ�ݣ��Ľ��ķ����ǣ������������Լ�������λ�õȣ�______________��
��1���ٷ�ֹSn2����������2�֣� ����ͨ©������©��������������2�֣���ȡ���һ��ϴ��Һ�������Թ��У��μ�ϡ�����AgNO3��Һ��������������֤��SnO�е�Cl����ϴ�Ӹɾ�����������ɫ����֤��SnO�е�Cl��δϴ�Ӹɾ���3�֣� ��cdabe��2�֣�
��2����Ũ���ᣨ2�֣��������ܣ�2�֣�
��D��2�֣������ڵĽ�������������Ӧ�����зų��������ȣ���ά�ָ÷�Ӧ�������У�2�֣�
����Ҫ�¶ȼƣ���װ��F��Ӧ����һ��װ�м�ʯ�ң����������ƹ��壩�ĸ���ܣ���U�ܣ���2�֣�
���������������1��������Sn2���ױ�����������SnCl2���ܺ����Sn�۵������Ƿ�ֹSn2����������
�ڲ�����еõ�©ҺB��������˵���ò����ǹ��ˣ�����õ��IJ����������ձ������ͨ©������©�������������������ӵļ���һ�����������ữ����������Һ�����Լ���SnO�е�Cl���Ƿ�ϴ�Ӹɾ��IJ���Ϊ��ȡ���һ��ϴ��Һ�������Թ��У��μ�ϡ�����AgNO3��Һ��������������֤��SnO�е�Cl����ϴ�Ӹɾ�����������ɫ����֤��SnO�е�Cl��δϴ�Ӹɾ���
�۴���Һ�еõ��������ȷ����������Ũ����.��ȴ�ᾧ�����ˡ�ϴ�ӡ����¸�������ȷ��˳����cdabe��
��2��������SnCl4�۵㣭33�棬�е�114.1�棬����ʪ��������ˮ�⣬���ͨ������������Ǹ���ģ���װ��C��Ӧ�����Լ�ΪŨ������������Ľṹ�ص��֪������E������Ϊ�����ܡ�
���������ڵĽ�������������Ӧ�����зų��������ȣ���ά�ָ÷�Ӧ�������У����Է�Ӧ��ʼ����SnCl4ʱ��������Ϩ��D���ľƾ��ơ�
�۷�Ӧ�������һ���¶ȷ�Χ֮�ڣ���Ҫ�¶ȼƣ���������֪SnCl4����ʪ�����㷢��ˮ�ⷴӦ��������Ӧ������Ӧ��װ��F��Ӧ����һ��װ�м�ʯ�ң����������ƹ��壩�ĸ���ܣ���U�ܣ��ɴﵽĿ�ġ�
���㣺���������Ʊ���ʵ�鷽������������Լ���ѧʵ������������й��ж�

 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д���������ʵ���ó�����Ӧ������ȷ����
| | ʵ����ʵ | ���� |
| A | �������������ܽ�������������Һ�� | �����������ڼ� |
| B | CO2��ˮ��Һ�ɵ��� | CO2�ǵ���� |
| C | SO2ͨ�����Ը��������Һ����Һ��ɫ | SO2��Ư���� |
| D | ���ֱ����������Ӧ�õ��Ȼ����������� | �����������Դ����� |
��ij�����Ļ����(��65%Cu��25%Al��8%Fe������Au��Pt)�Ʊ�����ͭ�������������·��������£�
��֪���ʿ�ʼ�����ͳ�����ȫʱpH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Cu(OH)2 |
| ������ʼʱpH | 2.7 | 4.1 | 8.3 |
| ������ȫʱpH | 3.7 | 5.4 | 9.8 |
��ش��������⣺
(1)���˲����õ��IJ���������________��
(2)�ڢٲ�Al�������ᷴӦ�����ӷ���ʽΪ___________________________��
�õ�����1����Ҫ�ɷ�Ϊ________��
(3)�ڢڲ���NaOH������ҺpH�ķ�ΧΪ________��
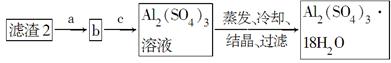
(4)�ɵڢ۲��õ�CuSO4��5H2O����IJ����ǽ���Һ2________��________�����ˡ�ϴ�ӡ����
(5)����ϴ�Ӻ������2��ȡAl2(SO4)3��18H2O���뽫a��b��c����������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | �� | �� | |
��1���ܡ��ݡ������Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��2���ߡ��ࡢ�����ۺ������������ǿ������˳����_________________________��
��3���ɱ��Т٢�Ԫ�ص�ԭ�Ӱ�1��1��ɵĻ������ϡ��Һ�ױ����ֽ⣬ͨ��ʹ�õĴ���Ϊ������ţ�____________________��
a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4
��4����ͼ�� A��F���ɲ����ϱ���Ԫ����ɵĵ��ʻ������A��B��CΪ���ʣ�ת����ϵ���£�

I.��BΪ��ɫ���壬AΪԭ�Ӱ뾶��С��ԭ����ɵĵ��ʣ�CΪ˫ԭ�ӷ�����ɵĵ��ʣ�E��ʹƷ����Һ��ɫ��
��F�ĵ���ʽΪ ��
��ʵ������ʼ�μӷ�Ӧ��B��������ɵ�B������ȣ�����ʼ�μӷ�Ӧ��A��C�����ʵ���֮���� ��
II.��DΪ����ɫ���壬��ɫ��ӦΪ��ɫ�����C��Ԫ�ص�ԭ���������������ڲ��������2����
�����й���D��˵����ȷ���� ������ĸ����
a.����ˮ�������Ϸ�Ӧ
b.���������ԣ����л�ԭ��
c.�Ⱥ����Ӽ����ֺ��Ǽ��Թ��ۼ�
d.��һ�ּ���������
���ö��Ե缫��F�ı�����Һ���е�⣬��������Ӧʽ�� ��