��Ŀ����
���ǵ����Ϻ����ḻ��ԭ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
��1��25��ʱ��0.1mol/LNH4NO3��Һ��ˮ�ĵ���̶� ������ڡ��������ڡ���С�ڡ��� 0.1mol/L NaOH��Һ��ˮ�ĵ���̶ȡ�
��2������0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������ϣ������Һ��2c(NH4+)��c(NO3��)��������Һ������Ũ���ɴ�С��˳���� ��
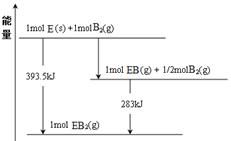
��3��������ʱ��(N2H4)Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ�����ⶨ16g������������Ӧ�зų�284kJ����������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4����ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��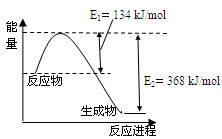
��֪��N2(g)��O2(g)��2NO(g) ��H����180kJ/mol
2NO (g)��O2(g)��2NO2(g) ��H����112.3kJ/mol
��Ӧ��2NO(g)��2CO(g) N2(g)��2CO2(g)�ġ�H�� ��
N2(g)��2CO2(g)�ġ�H�� ��
��1������ ��2��c(NO3��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)
��3��2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol��4����760.3kJ/mol
������������� ��1���ᡢ������ˮ�ĵ��롢��ˮ����δٽ�ˮ�ĵ��롣
��2��0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������Ϻ�c(Na+)=0.05mol/L��c(NO3-)=0.1mol/L����2c(NH4+)��c(NO3��)��c(NH4+)>0.05mol/L������غ�ʽΪc(Na+)+c(NH4+)+c(H+)=c(NO3-)+c(OH-)����c(Na+)��c(NO3-)��c(NH4+)��c(OH��)��c(H+)������c(NO3��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)��
��3��2N2H4��2NO2��3N2��2H2O��16gN2H4Ϊ0.5mol������2molN2H4��Ӧ����1136kJ��
��4����ͼ��ɵ�NO2(g)+CO(g) CO2(g)+NO(g) ��H����234kJ/mol���ɸ�˹���ɵ�2NO(g)��2CO(g)
CO2(g)+NO(g) ��H����234kJ/mol���ɸ�˹���ɵ�2NO(g)��2CO(g) N2(g)��2CO2(g)�ġ�H=����234��2+180+112.3��kJ/mol=��760.3kJ/mol��
N2(g)��2CO2(g)�ġ�H=����234��2+180+112.3��kJ/mol=��760.3kJ/mol��
���㣺 ˮ�ĵ��� ����Ũ�ȱȽ� �Ȼ�ѧ����ʽ ��˹����

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�(14��)CO2��һ����Ҫ���������壬�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
��1�����ʯ��ʯīȼ�շ�Ӧ�е������仯��ͼ��ʾ��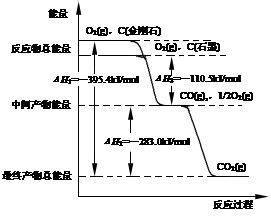
����ͨ��״���£����ʯ��ʯī�У� ������ʯ����ʯī�������ȶ���ʯī��ȼ����Ϊ kJ��mol��1��
��ʯī��CO2��Ӧ����CO���Ȼ�ѧ����ʽ�� ��
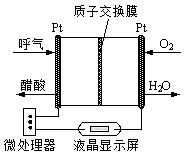
��2�����õ绯ѧ���ɽ�CO2ת��Ϊ���顣��д������������ˮ��Һ�������ʱ����ת���ĵ缫��Ӧ����ʽ ��
��3��CO2Ϊԭ�ϻ��ɺϳɶ������ʡ���ҵ�ϳ���CO2(g) ��H2(g)Ϊԭ�Ϻϳ��Ҵ���
����֪��H2O(l)=H2O(g) ��H=+44kJ��mol��1
CO(g)+H2O(g) CO2(g)+H2(g) ��H=��41.2kJ��mol��1
CO2(g)+H2(g) ��H=��41.2kJ��mol��1
2CO(g)+4H2 (g)  CH3CH2OH(g)+H2O(g) ��H= ��256.1kJ��mol��1��
CH3CH2OH(g)+H2O(g) ��H= ��256.1kJ��mol��1��
��2CO2(g)+6H2(g)  CH3CH2OH(g)+3H2O(l) ��H= ��
CH3CH2OH(g)+3H2O(l) ��H= ��
����ͼ��һ�����̵���Ϊԭ�Ϻϳ��Ҵ��Ĺ���ԭ��ʾ��ͼ��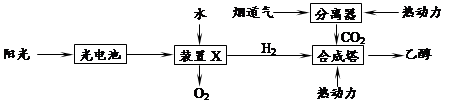
���������̵ķ���������˵����ȷ���� ��
| A�����������ٰ���4����ʽ������ת�� |
| B��װ��X��������ӦΪ��2H2O��4e��=4H++O2�� |
| C���ϳ����������Ҵ��ķ�Ӧ�ǻ��Ϸ�Ӧ |
| D�����������������ɫ��ѧ˼�� |
ͼa��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��ͼb�Ƿ�Ӧ�е�CO��NO��Ũ����ʱ��仯��ʾ��ͼ������ͼ��ش��������⣺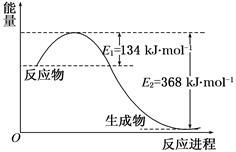
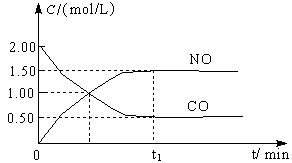
a b
(1)д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
(2)�ӷ�Ӧ��ʼ��ƽ�⣬��NO2Ũ�ȱ仯��ʾƽ����Ӧ����v(NO2)�� ��
(3)���¶��¸÷�Ӧ��ƽ�ⳣ��K= ���¶Ƚ��ͣ�K ����������С�����䡱��
(4)�����¶Ⱥ��ݻ���ͬ�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ�ⅼ���й��������±���
| �� �� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol NO2 1 mol CO | 2 mol NO 2 mol CO2 | 1 mol NO2��1 mol CO 1 mol NO��1 mol CO2 |
| ƽ��ʱc(NO) /mol��L-1 | 1.5 | 3 | m |
| �����仯 | �ų�a kJ | ����b kJ | �ų�c kJ |
| CO��NO��ת���� | ��1 | ��2 | ��3 |
��1+��2= �� a+b/2= ,m=
��������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ���ȼ�ղ���ΪSO2��Fe2O3��
��1����֪1g FeS2��ȫȼ�շų�7.1kJ���������ʾFeS2��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ��
______________________________________________________________��
��2����0.050molSO2(g)��0.030molO2(g)�����ݻ�Ϊ1L���ܱ������У���Ӧ��2SO2(g)��O2(g) 2SO3(g) ��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol/L����������·�Ӧ��ƽ�ⳣ��K����ֵΪ___________,SO2��ƽ��ת����Ϊ__________��
2SO3(g) ��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol/L����������·�Ӧ��ƽ�ⳣ��K����ֵΪ___________,SO2��ƽ��ת����Ϊ__________��
��3�����÷�Ӧ����ƽ��״̬ʱ����ʹƽ��������Ӧ�����ƶ��ҷ�Ӧ���ʼӿ죬���д�ʩ���е��� ��������ĸ��
| A����ƽ�������г���Ar | B����ƽ�������г���O2 |
| C���ı䷴Ӧ�Ĵ��� | D�����ͷ�Ӧ���¶� |
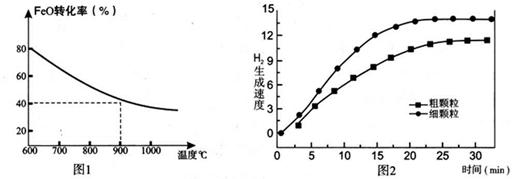
 2SO3(g) ��H<0 �� SO2��ת��������ʼ�¶�T1=673K���淴Ӧʱ�䣨t���ı仯����ͼ�������������䣬���ı���ʼ�¶�ΪT2=723K������ͼ�л����¶�T2��SO2��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
2SO3(g) ��H<0 �� SO2��ת��������ʼ�¶�T1=673K���淴Ӧʱ�䣨t���ı仯����ͼ�������������䣬���ı���ʼ�¶�ΪT2=723K������ͼ�л����¶�T2��SO2��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��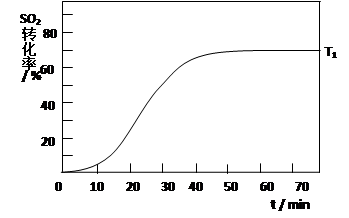

 BaCO3(s)+SO42��(aq)
BaCO3(s)+SO42��(aq)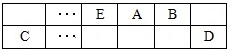

 Fe3O4(s)+4H2(g) ��H=akJ/mol ��I��
Fe3O4(s)+4H2(g) ��H=akJ/mol ��I��