��Ŀ����
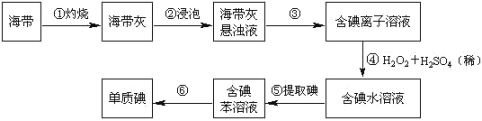
����Ŀ���赥�ʼ��仯����Ӧ�÷�Χ�ܹ㡣���ȹ��飨SiHCl3����ԭ����Ŀǰ��ҵ�Ƹߴ��ȹ����Ҫ������������������ͼ��
![]()
��������������б��⣺
��1������Ԫ�����ڱ���λ��____________�����������____________��������ͬ�ĵ��ӡ�
��2�������̬�⻯��ΪSiH4����ռ乹��Ϊ____________�����ȹ�����ijЩԪ������������Ӧˮ���������____________![]() ____________
____________![]() �ѧʽ
�ѧʽ![]() ��
��
��3��д�����ȹ�����������Ӧ�Ļ�ѧ��Ӧ����ʽ��____________��
��4����Ȼ���й���������࣬�ṹ���ӣ�ͨ���ö�������ͽ������������ʽ����ʾ����ɡ�������ʯ��KAlSi3O8������������ʽΪ��____________��
���𰸡��������ڢ�A�� 2 �������� HClO4 H2SiO3 ![]() K2O��Al2O3��6SiO2
K2O��Al2O3��6SiO2
��������
��1���ɹ�ԭ�ӽṹʾ��ͼΪ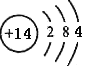 ��֪��Ԫ����Ԫ�����ڱ���λ��Ϊ�������ڣ��ڢ�A�壻��ԭ�������4�����ӷֲ���3s��3p����ϣ�������ͬ���ʴ�Ϊ���������ڢ�A�壻2��
��֪��Ԫ����Ԫ�����ڱ���λ��Ϊ�������ڣ��ڢ�A�壻��ԭ�������4�����ӷֲ���3s��3p����ϣ�������ͬ���ʴ�Ϊ���������ڢ�A�壻2��
��2�������̬�⻯��Ϊ�軯�⣬�軯������й�ԭ�ӵļ۲���Ӷ���Ϊ4���¶Ե�����Ϊ0�����ԭ��Ϊsp3�ӻ����軯����ӹ���Ϊ�������壻���ȹ���������Ԫ�صķǽ�����ǿ�ڹ�Ԫ�أ��ǽ�����Խǿ������������Ӧˮ���������Խǿ���ʴ�Ϊ���������壻HClO4��H2SiO3��
��3�����ȹ�����������Ӧ���ɹ���Ȼ��⣬��Ӧ�Ļ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��4������ʯΪ�����Σ��εĻ�ѧʽΪKAlSi3O8������ԭ�Ӹ����Ȳ����ԭ���֪���������ʽΪ��K2O��Al2O3��6SiO2���ʴ�Ϊ��K2O��Al2O3��6SiO2��

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
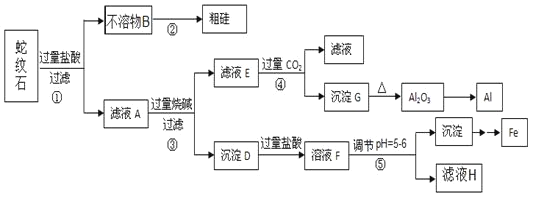
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�����Ŀ������ʯ����Կ�����MgO��![]() ��
��![]() ��
��![]() ��ɡ�ijʵ��С����������������ֱ��Ƶõ���Al��Fe��Mg��Si��
��ɡ�ijʵ��С����������������ֱ��Ƶõ���Al��Fe��Mg��Si��
�й��������������pH���±���
���������� | �������↑ʼ����ʱ��pH | ����������ȫ����ʱ��pH |
| 1.9 | 3.2 |
| 9.4 | 11.6 |
��1������ʯ������������þ�������⼸��Ԫ�صļ����ӵİ뾶��С�����˳��_____����ҺA�к��е��������� _________��
��2����Ԫ�������ڱ��е�λ��______��������̼�ĵ���ʽ___________��
��3���������з�Ӧ�Ļ�ѧ����ʽΪ ____�������������ɳ���G�����ӷ���ʽΪ _____��
��4���������е���pH��![]() ʱ�������õ����Լ�
ʱ�������õ����Լ�![]() �����
�����![]() ____________��
____________��
a��NaOH b����ˮ c��MgO d��Mg��OH��2
��5������ҺH��ȡ����Mg���������£�
![]()
������ұ��þ�ķ�����__________���ڸ����HCl�����м���MgCl2��6H2O��ȡ��ˮ�Ȼ�þ��ԭ����__________��