��Ŀ����
����Ŀ���Ȼ���(HSO3Cl)���Ǿ����ǰ�ҩ������������Ҫ��Ӧ�á����³�ѹ���Ȼ���Ϊ��ɫ��״Һ�壬�е�ԼΪ152�棬��ʪ�Ժ�ʴ�Լ�ǿ���ڿ����з��̡�ѧϰС����ʵ������SO3��HCl���Ʊ�HSO3Cl���ⶨ��Ʒ���ȡ��������ʵ��(�г�װ����ȥ)����ش��������⣺

��1������m������Ϊ___��
��2����֪��HSO3Cl����Ԫ��Ϊ��6�ۣ�Oԭ�Ӻ�Clԭ�ӵ�����������8�����ȶ��ṹ����HSO3Cl�еĻ�ѧ��Ϊ___ (�������Ӽ����������Լ��������Ǽ��Լ���)��
��3��SO3����������������Ũ���Ṳ���Ʊ���������Ӧ�Ļ�ѧ����ʽΪ___��
��4��װ��B������Ϊ___��������n��֪�Ʊ�HSO3Cl�ķ�ӦΪ___ (�������ȷ�Ӧ���������ȷ�Ӧ��)��
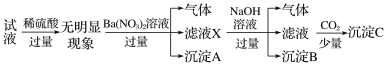
��5��HSO3Cl���ȵIJⶨ(����m�еõ���HSO3Cl�г�����������SO3)��
i.ȡ25.0g��Ʒ����ˮ�У����������Ba(NO3)2��Һ��ַ�Ӧ���ˡ�
ii.����Һ�м��������AgNO3��Һ�����ˡ�ϴ�ӡ�����������������ó���AgCl������Ϊ28.7g��
��HSO3Cl��ˮ������Ӧ�Ļ�ѧ����ʽΪ___��
�ڲ�ƷHSO3Cl�Ĵ���Ϊ___��
���𰸡�������ƿ ���Լ� P2O5��3H2SO4��Ũ��![]() 3SO3����2H3PO4 ��ֹ�����е�ˮ������ �뷴Ӧװ��A�У������ջӷ�������HCl���� ���ȷ�Ӧ HSO3Cl+H2O=HCl+H2SO4 93.2%
3SO3����2H3PO4 ��ֹ�����е�ˮ������ �뷴Ӧװ��A�У������ջӷ�������HCl���� ���ȷ�Ӧ HSO3Cl+H2O=HCl+H2SO4 93.2%
��������
��1������m������Ϊ������ƿ���ʴ�Ϊ��������ƿ��
��2��HSO3Cl����Ԫ��Ϊ��6�ۣ�Oԭ�Ӻ�Clԭ�ӵ�����������8�����ȶ��ṹ���ṹʽΪ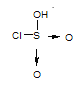 ����HSO3Cl�еĻ�ѧ��Ϊ���Լ� ���ʴ�Ϊ�����Լ���
����HSO3Cl�еĻ�ѧ��Ϊ���Լ� ���ʴ�Ϊ�����Լ���
��3��SO3����������������Ũ���Ṳ���Ʊ���������Ӧ�Ļ�ѧ����ʽΪP2O5��3H2SO4��Ũ��![]() 3SO3����2H3PO4���ʴ�Ϊ��P2O5��3H2SO4��Ũ��
3SO3����2H3PO4���ʴ�Ϊ��P2O5��3H2SO4��Ũ��![]() 3SO3����2H3PO4��
3SO3����2H3PO4��
��4��HSO3Cl��ˮ����ҷ�Ӧ��������m���ݳ���HCl��Ⱦ������װ��B������Ϊ��ֹ�����е�ˮ�������뷴Ӧװ��A�У������ջӷ�������HCl���塣����n����������������HSO3Cl����֪�Ʊ�HSO3Cl�ķ�ӦΪ���ȷ�Ӧ���ʴ�Ϊ����ֹ�����е�ˮ�������뷴Ӧװ��A�У������ջӷ�������HCl���壻���ȷ�Ӧ��
��5����HSO3Cl��ˮ������Ӧ�Ļ�ѧ����ʽΪHSO3Cl+H2O=HCl+H2SO4��
�ڸ���������Ϣ��HSO3Cl~AgCl������HSO3Cl�Ĵ���Ϊ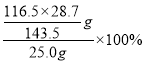 =93.2%��
=93.2%��
�ʴ�Ϊ��HSO3Cl+H2O=HCl+H2SO4��93.2%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��SO2�㷺����ҽҩ�����Ṥҵ�������շ����е�SO2�������·�����
������ | �ü�ʽ������Al2(SO4)x(OH)y��Һ���ո���SO2 |
������ | ��Fe2+��Fe3+���£��ÿ���(O2)��SO2����ΪH2SO4 |
(1)������Ĺ������¡�
�� �Ʊ�Al2(SO4)x(OH)y����Al2(SO4)3��Һ�м���CaO��ĩ����pH��3.6�� CaO��������______
�� ���գ�Al2(SO4)x(OH)y����SO2��IJ�����______(д��ѧʽ)��
�� ���������Ȣ��в������SO2��Al2(SO4)x(OH)y������
(2)�������У���Fe2+���£�SO2��O2��H2O����H2SO4�Ļ�ѧ����ʽ��______��
(3)�������У�Fe2+�Ĵ����̿ɱ�ʾ���£�
����2Fe2++O2+SO2=2Fe3++SO42-
��������
�� д���������ӷ���ʽ��______��
�� ����ʵ�鷽����֤ʵ���������̡���ʵ�鷽������������
a.��FeCl2��Һ����KSCN���ޱ仯
b.��FeCl2��Һͨ������SO2������KSCN����ɫ��졣
c.ȡb����Һ��______��
(4)�������У����������õζ����ⶨ�����в���SO2�ĺ�������V L(�ѻ���Ϊ��״��)�����е�SO2��1%��H2O2��ȫ���գ�����Һ����ͼ��ʾװ�õζ���������a mL c mol/L NaOH��Һ��
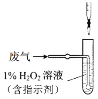
��H2O2����SO2�Ļ�ѧ����ʽ______��
�� �����в���SO2���������Ϊ______��
����Ŀ��(1)�һ��嶡����(��ETBE��ʾ)��һ�����������ĸ�����ֵ���͵��ͼ������Ҵ����춡ϩ(��IB��ʾ)�ڴ���HZSM��5���ºϳ�ETBE����Ӧ�Ļ�ѧ����ʽΪ��C2H5OH(g)+IB(g)=ETBE(g) ��H���ش��������⣺
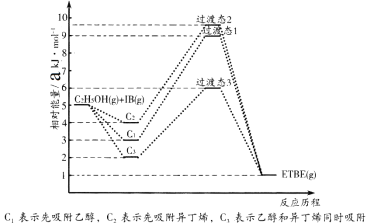
��Ӧ�ﱻ����HZSM��5������˳���뷴Ӧ���̵Ĺ�ϵ��ͼ��ʾ���÷�Ӧ�ġ�H=__________ kJ��mol-1����Ӧ���̵�����;����________(��C1��C2��C3)��
(2)���������Դ�ǵ����о���һ���ȵ����⡣������(CH3OCH3)��δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ����(ѹ��2.0��10.0Mpa���¶�230��280��)�������з�Ӧ��
��Ӧ����CO(g)+2H2(g)CH3OH(g) ��H1=-99kJ��mol1
��Ӧ����2CH3OH(g)CH3OCH3(g)+H2O(g) ��H2=-23.5kJ��mol1
��Ӧ����CO(g)+H2O(g)CO2(g)+H2(g) ��H3=-41.2kJ��mol1
���ڸ������£�����Ӧ1����ʼŨ�ȷֱ�Ϊ��c(CO)=0.6mol��L1��c(H2)=1.4mol��L1��8min��ﵽƽ�⣬CO��ת����Ϊ50%����8min��H2��ƽ����Ӧ����Ϊ__________��
����t��ʱ����Ӧ2��ƽ�ⳣ��Ϊ400�����¶��£���1L���ܱ������м���һ���ļ״�����Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
c(mol��L1) | 0.46 | 1.0 | 1.0 |
��ʱ��v��___v��(���������������)��ƽ��ʱc(CH3OCH3)�����ʵ���Ũ����___��
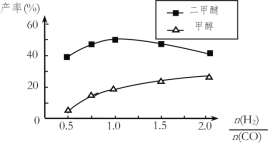
�۴���Ӧ�ҵ��ܷ�Ӧ3CO(g)+3H2(g)CH3OCH3(g)+CO2(g)��CO��ƽ��ת������(CO)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��ͼ��X����___(��¶ȡ���ѹǿ��)����L1___L2(���������������)��

���ڴ�����������ͬʱ����������Ӧ������������ʼͶ�ϱ�![]() �ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��______________��
�ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��______________��