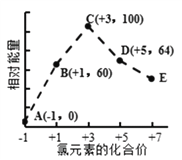
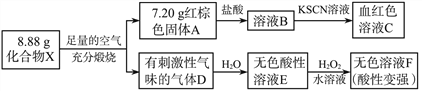
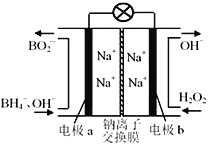
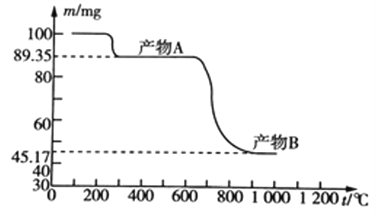
��Ŀ����
����Ŀ��ij�¶��£���[H��]��1��10��6 mol��L��1������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��[H��]��1��10��3mol��L��1�����жԸ���Һ����������ȷ������ ��
A. ���¶ȸ���25 ��
B. ������Һ�У���ˮ���������H����Ũ��Ϊ1��10��11mol��L��1
C. ����NaHSO4��������ˮ�ĵ���
D. ���¶��£���NaHSO4��Һ��ijpH��11��Ba(OH)2��Һ��Ϻ���Һ�����ԣ������ĵ�NaHSO4��Һ��Ba(OH)2��Һ�������Ϊ100��1
���𰸡�B
��������A.25��ʱ[H��]��1��10��7 mol��L��1��[H��]��1��10��6 mol��L��1˵���ٽ���ˮ�ĵ��룬��T��25�棬ѡ��A��ȷ��B��ij�¶��£���[H��]��1��10��6 mol��L��1������ˮ����Kw=1��10��12mol2��L��2������ҺΪǿ����Һ��[H��]��1��10��3mol��L��1��������������Ũ��=![]() mol/L=10-10mol/L������Һ��ˮ�������������Ũ�ȵ�������������Ũ��10-10mol/L����ˮ���������H����Ũ��Ϊ10-10mol/L��ѡ��B����ȷ��C. ����NaHSO4���壬��ˮ�е���������ӡ������Ӻ���������ӣ�������Ũ����������ˮ�ĵ��룬ѡ��C��ȷ��D�����¶��£���NaHSO4��Һ[H��]��1��10��3mol��L��1,ijpH��11��Ba(OH)2��Һ[OH-]��1��10��1mol��L��1,��Ϻ���Һ�����ԣ���1��10��3mol��L��1��V����1��10��1mol��L��1��V���������ĵ�NaHSO4��Һ��Ba(OH)2��Һ�������Ϊ100��1��ѡ��D��ȷ����ѡB��
mol/L=10-10mol/L������Һ��ˮ�������������Ũ�ȵ�������������Ũ��10-10mol/L����ˮ���������H����Ũ��Ϊ10-10mol/L��ѡ��B����ȷ��C. ����NaHSO4���壬��ˮ�е���������ӡ������Ӻ���������ӣ�������Ũ����������ˮ�ĵ��룬ѡ��C��ȷ��D�����¶��£���NaHSO4��Һ[H��]��1��10��3mol��L��1,ijpH��11��Ba(OH)2��Һ[OH-]��1��10��1mol��L��1,��Ϻ���Һ�����ԣ���1��10��3mol��L��1��V����1��10��1mol��L��1��V���������ĵ�NaHSO4��Һ��Ba(OH)2��Һ�������Ϊ100��1��ѡ��D��ȷ����ѡB��

����Ŀ����.��ϩ�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������ϩΪ��Ҫԭ�Ϻ���Ҫ���л�������·������ͼ��ʾ����ش��������⡣
![]()
(1)A�������������ŵ�������____��
(2)��Ӧ�۵Ļ�ѧ����ʽ��____��
(3)���������У�����ͨ����ϩ�ӳɷ�Ӧ�õ�����____������ţ���
a. CH3CH3 b. CH3CHCl2 c. CH3CH2Br
��.���ѿ�������ơ�
(1)��������֭�������ǵķ����ǣ������мӼ�������ԣ��ټ������Ʊ���Cu��OH��2�����ȣ���������____��
(2)���Ѿ��ܷⴢ�����������������ζ��������Ҳ����ͨ����ѧʵ�����Ʊ���ʵ��������ͼ��ʾװ���Ʊ�����������

���Թ�a���������������Ļ�ѧ����ʽ��____��
��ʵ�鿪ʼʱ���Թ�b�еĵ��ܲ�����Һ���µ�ԭ����____��
(3)�л���E��̼���⡢������Ԫ����ɣ����������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������EΪ��ɫճ��Һ�壬������ˮ��Ϊ�о�E�������ṹ������������ʵ�飺
�ٳ�ȡE4.5g������ʹ�������������ܶ�����ͬ������H2��45���� | ���л���E����Է�����Ϊ��__�� |
�ڽ���9.0gE��������O2���ȼ�գ���ʹ���������ͨ����ʯ�ҡ���ˮ����ͭ��ĩ������ʯ��ˮ�����ּ�ʯ������14.2g������ͭ��ĩû�б�����ʯ��ˮ����10.0g��ɫ�������ɣ������صļ�ʯ���м��������������4.48L��ɫ��ζ���壨��״������ | ��9.0g�л���E��ȫȼ��ʱ�������㣺����CO2��Ϊ____ mol�� |
�۾�������ײⶨ��֤ʵ���к����ǻ����Ȼ����� |
��д��E��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ__________��