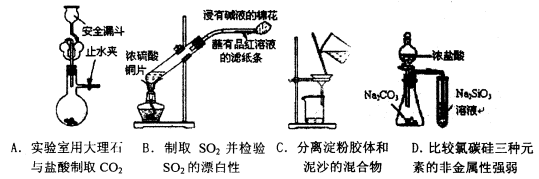
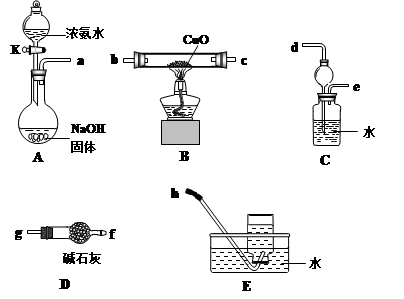
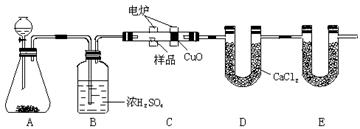
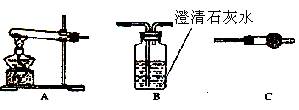
��Ŀ����
ijͬѧ��ͬ����Ԫ�����ʵݱ����ʵ��ʱ���Լ������һ��ʵ�鷽��������¼���й�ʵ���������±�������±���ʵ������II������A��B��C��������ѡ����ʵ�鷽����I������1��2��3��������Ӧ��ʵ����������𰸣�III���С�
ͨ������ʵ�������ͬ���ڵ���ЩԪ��(��Ԫ�ط���)________________����ʵ�������Եó��Ľ�����______________________________________��
| ʵ�鷽����I�� | ʵ������II�� | �𰸣�III�� | |
| I | II | ||
| 1����ɰֽ�����þ�����ˮ��Ӧ������ӦҺ�еμӷ�̪ | A������ˮ�棬���ҷ�Ӧ���ų����壬�۳�һ��С����ˮ���������ƶ�����֮��ʧ����Һ���ɫ�� | 1 | |
| 2�������Ƶ�H2S������Һ�еμ����Ƶ���ˮ | B�������������壬���ڿ�����ȼ�գ���Һ���dz��ɫ | 2 | |
| 3��������з�̪����ˮ��Ӧ | C����Ӧ��ʮ�־��ң���������������ڿ�����ȼ�� | 3 | |
| 4��þ����2mol/L�����ᷴӦ | D�����ҷ�Ӧ����������������ڿ�����ȼ�� | 4 | |
| 5��������2mol/L�����ᷴӦ | E�����ɰ�ɫ��״�������̶�������ʧ | 5 | |
| 6����AlCl3��Һ�еμ�NaOH��Һ������ | F�����ɵ���ɫ���� | 6 | �� |
| �𰸣�III�� | |
| I | II |
| 1 | B |
| 2 | F |
| 3 | A |
| 4 | D |
| 5 | C |
| 6 | E |
ͬһ���ڣ�����ԭ�������ĵ�����Ԫ�صĽ����Լ������ǽ�������ǿ����2�֣�
�������������������һЩ�Ա�ʵ�飬��Ȼ��Ϊ��̽��Ԫ�صĵݱ���ɡ�A����Һ���ɫ������Ϊ���ɼ��Ե�NaOH��B��C��D�п�ȼ�յ�����ΪH2��E�а�ɫ��״����Ϊ����������F�е���ɫ������S���ʡ�
�������Ի�ѧʵ��Ŀ���������ĸ߿��ص㣬�����ڱ�����Ӧע��Ի�ѧ������ʹ�á���ѧʵ������ʵ�鰲ȫ���������ʵ��Ʊ����ռ��ȵ����֪ʶ�Ļ��ۡ��ѶȽϴ�

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д� ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ