��Ŀ����
 �������������������бز����ٵ���Ҫ��������ͼ����
�������������������бز����ٵ���Ҫ��������ͼ������1����Ȼ��һ�����������ȶ�����ԭ�ӣ��ֱ���54Fe��56Fe��57Fe��58Fe������58Feԭ����������������֮��Ϊ
��2����ԭ������
��3������ͬ���ڵ�����Ԫ���У�����������Ӧˮ�����У�������ǿ�Ļ�������������ǿ�Ļ�����Ļ�ѧ��Ӧ����ʽΪ
��4��Ҫ����Ƭ��пƬ��ֱ����Դ�͵��������Ƭ��п��ʵ�飬��ƬӦ����
��5���������ƣ�Na2FeO4����ˮ����������ʹ�õ�һ�����;�ˮ�������������Աȸ�����ظ�ǿ�������ڷ�Ӧ�б���ԭΪFe3+����ƽ��ȡ�������ƵĻ�ѧ����ʽ��
��6��0.03mol�����ӵ�������HNO3�У����ȣ�����ȫ�ܽ⣬������NO��NO2�Ļ�����干1.12L����״��������ʢ�д����������������ˮ�У�ͨ���״����һ�������O2��ǡ��ʹ����ȫ������ˮ����HNO3����ͨ��O2�����
��������1������ԭ��������26��Ԫ�صĽ���ƽ�����ԭ������Ϊͬλ�ص����������Ը�ͬλ��ԭ������Ȼ����ռԭ�Ӹ����İٷ�����
��2����ԭ����7���ܼ����������9��ԭ�ӹ����
��3������ͬ���ڵ�����Ԫ���У�������ǿ�Ļ�������������ǿ�Ļ�����ֱ�ΪKOH��HBrO4��
��4�����ƽ��������������Ʋ�����������������Һ��
��5���������غ㶨�ɼ������غ�����ƽ��ѧ��Ӧ��FeԪ�صĻ��ϼ���+3������Ϊ+6�ۣ�ClԪ�صĻ��ϼ�ӦΪ0����Ϊ-1�ۣ������Ʊ���ԭ��IJ���Fe3+�ܷ���ˮ�ⷴӦ���������������壬��������������������ԣ�
��6�����ݵ����غ���㣬��֪HNO3
NO��NO2
HNO3����Ӧǰ��HNO3�����ʵ������䣬�����ϼ۱仯��ֻ����������������ʧȥ������Ŀ����O2�õ����ӵ���Ŀ���Դ˽��м��㣮
��2����ԭ����7���ܼ����������9��ԭ�ӹ����
��3������ͬ���ڵ�����Ԫ���У�������ǿ�Ļ�������������ǿ�Ļ�����ֱ�ΪKOH��HBrO4��
��4�����ƽ��������������Ʋ�����������������Һ��
��5���������غ㶨�ɼ������غ�����ƽ��ѧ��Ӧ��FeԪ�صĻ��ϼ���+3������Ϊ+6�ۣ�ClԪ�صĻ��ϼ�ӦΪ0����Ϊ-1�ۣ������Ʊ���ԭ��IJ���Fe3+�ܷ���ˮ�ⷴӦ���������������壬��������������������ԣ�
��6�����ݵ����غ���㣬��֪HNO3
| �� |
| ���� |
����⣺��1��������=������-������=58-26=32����������������֮��Ϊ32-26=6��Ԫ�صĽ���ƽ�����ԭ������Ϊͬλ�ص����������Ը�ͬλ��ԭ������Ȼ����ռԭ�Ӹ����İٷ�������ȣ����ʴ�Ϊ��6����ȣ�
��2�����ĵ����Ų�ʽ��1s22s22p63s23p63d64s2����ԭ����7���ܼ��������ΪM�㣬��9��ԭ�ӹ�����ʴ�Ϊ��7��9��
��3������ͬ���ڵ�����Ԫ���У�����������Ӧˮ�����У�������ǿ�Ļ�������������ǿ�Ļ�����ֱ�ΪKOH��HBrO4����ѧ��Ӧ����ʽΪKOH+HBrO4=H2O+HBrO4���ʴ�Ϊ��KOH+HBrO4=H2O+HBrO4��
��4�����ƽ������������������������Ʋ�����������������Һ��ZnCl2���������Һ���ʴ�Ϊ������ZnCl2��Zn ������Һ�����ԣ���
��5��FeԪ�صĻ��ϼ���+3������Ϊ+6�ۣ�ClԪ�صĻ��ϼ�ӦΪ0����Ϊ-1�ۣ��������غ㶨�ɼ������غ��֪��ƽ��ѧ��ӦΪ2Fe��NO3��3+16NaOH+3Cl2=2Na2FeO4+6NaNO3+6NaCl+8H2O�������Ʊ���ԭ��IJ���Fe3+�ܷ���ˮ�ⷴӦ���������������壬��������������������ԣ�
�ʴ�Ϊ��2��16��3��2��6��6�� 8��Fe3+ˮ�⣬������Һ�е����ʣ�
��6��0.03mol��Ӧʱʧȥ������ĿΪ3��0.03mol=0.09mol��
��Ӧ��������ΪHNO3
NO��NO2
HNO3����Ӧǰ��HNO3�����ʵ������䣬�����ϼ۱仯��ֻ����������������ʧȥ������Ŀ����O2�õ����ӵ���Ŀ�������������������ʵ���Ϊn��O2��
=0.0225mol��V��O2��=0.0225mol��22.4l/mol=0.504l������ͨ��O2�����Ϊ0.504l���ʴ�Ϊ��0.504L��
��2�����ĵ����Ų�ʽ��1s22s22p63s23p63d64s2����ԭ����7���ܼ��������ΪM�㣬��9��ԭ�ӹ�����ʴ�Ϊ��7��9��
��3������ͬ���ڵ�����Ԫ���У�����������Ӧˮ�����У�������ǿ�Ļ�������������ǿ�Ļ�����ֱ�ΪKOH��HBrO4����ѧ��Ӧ����ʽΪKOH+HBrO4=H2O+HBrO4���ʴ�Ϊ��KOH+HBrO4=H2O+HBrO4��
��4�����ƽ������������������������Ʋ�����������������Һ��ZnCl2���������Һ���ʴ�Ϊ������ZnCl2��Zn ������Һ�����ԣ���
��5��FeԪ�صĻ��ϼ���+3������Ϊ+6�ۣ�ClԪ�صĻ��ϼ�ӦΪ0����Ϊ-1�ۣ��������غ㶨�ɼ������غ��֪��ƽ��ѧ��ӦΪ2Fe��NO3��3+16NaOH+3Cl2=2Na2FeO4+6NaNO3+6NaCl+8H2O�������Ʊ���ԭ��IJ���Fe3+�ܷ���ˮ�ⷴӦ���������������壬��������������������ԣ�
�ʴ�Ϊ��2��16��3��2��6��6�� 8��Fe3+ˮ�⣬������Һ�е����ʣ�
��6��0.03mol��Ӧʱʧȥ������ĿΪ3��0.03mol=0.09mol��
��Ӧ��������ΪHNO3
| �� |
| ���� |
| 0.09mol |
| 4 |
���������⿼������ԭ�ӽṹ����ơ�������ԭ��Ӧ����ƽ�ͼ��㣬�Ѷ��еȣ�����HNO3
NO��NO2
HNO3����Ӧǰ��HNO3�����ʵ������䣬�����ϼ۱仯��ֻ����������������ʧȥ������Ŀ����O2�õ����ӵ���Ŀ�ǽ���Ĺؼ���
| �� |
| ���� |

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ


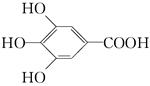 ��ûʳ��������īˮ��Ҫ������________����������(�����)��
��ûʳ��������īˮ��Ҫ������________����������(�����)��