��Ŀ����
�������������������бز����ٵ���Ҫ������

��1����Ȼ��һ�����������ȶ�����ԭ�ӣ��ֱ���54Fe��56Fe��57Fe��58Fe������58Feԭ����������������֮��Ϊ___________��
������Ԫ�صĽ�����������ļ���ʽΪ��54��a1%+56��a2%+57��a3%+58��a4%������a1%��a2%������ָ��ͬλ�ص�____________________��
��2����ԭ������______��������ͬ�ĵ��ӣ���ԭ�Ӵ����ĵ�������_____�ֲ�ͬ����չ����
��3������ͬ���ڵ�����Ԫ���У�����������Ӧˮ�����У�������ǿ�Ļ�������������ǿ�Ļ�����Ļ�ѧ��Ӧ����ʽΪ________________________________��
��4��Ҫ����Ƭ��пƬ��ֱ����Դ�͵��������Ƭ��п��ʵ�飬��ƬӦ����_________�����������Һ��____________________��
��5���������ƣ�Na2FeO4����ˮ����������ʹ�õ�һ�����;�ˮ�������������Աȸ�����ظ�ǿ�������ڷ�Ӧ�б���ԭΪFe3+����ƽ��ȡ�������ƵĻ�ѧ����ʽ��
___Fe(NO3)3 + ___NaOH + ___Cl2 ��___Na2FeO4 + ___NaNO3 + ___NaCl + ___H2O
�������Ƴ���������ɱ���⣬��������ˮ�е��������ԭ����______________________��
��6��0.03mol�����ӵ�������HNO3�У����ȣ�����ȫ�ܽ⣬������NO��NO2�Ļ�����干1.12L����״��������ʢ�д����������������ˮ�У�ͨ���״����һ�������O2��ǡ��ʹ����ȫ������ˮ����HNO3����ͨ��O2�����________________L��
��1��6 ��ȣ���1�֣�
��2��7 9����1�֣�
��3��KOH + HBrO4 ��H2O + HBrO4 ��2�֣�
��4���� ZnCl2��Zn ������Һ�����ԣ� ����1�֣�
��5��2 16 3 2 6 6 8 �� Fe3+ˮ�⣬������Һ�е����ʣ���1�֣�
��6��0.504L�� 2�֣�
��������
�����������1��58Feԭ���У�������Ϊ26��������Ϊ32������֮��Ϊ6��a1%��a2%������ָ��ͬλ�ص�����Ȼ���е�ԭ�Ӱٷֺ���������ȡ�
��2��������ԭ�ӵĺ�������Ų�ʽ��֪����7��������ͬ�ĵ��ӣ���ԭ�Ӵ����ĵ��Ӽ���3���Ӳ㣬��������9�ֲ�ͬ����չ����
��3������ͬ���ڵ�����Ԫ���У�����������Ӧˮ�����У�������ǿ�Ļ�����������������������ǿ�Ļ����� HBrO4 ����Ӧ�Ļ�ѧ��Ӧ����ʽΪKOH + HBrO4 ��H2O + HBrO4 ��
��4��Ҫ����Ƭ��пƬ��ֱ����Դ�͵��������Ƭ��п��ʵ�飬��ƬӦ������ �����������Һ��ZnCl2��Zn ������Һ�����ԣ���
��5�����ݻ��ϼ�����������ȣ�������ƽ���������Ƴ���������ɱ���⣬��������ˮ�е��������ԭ���� Fe3+ˮ�⣬������Һ�е����ʡ�
��6��NO2��NO�Ļ������Ϊ0.05mol���� O2��Ϻ�ͨ��ˮ�У���������ǡ����ȫ��ˮ�����������ᣬ�����ṩ�ĵ������ʵ�������������õ�����ӵ������ʵ�������ʧȥ���ӱ�����������ӣ�ʧ����Ϊ0.09mol�����������ʵ���Ϊ0.09/4mol,���Ϊ0.504L��
���㣺���⿼�������ӵ��Ų���������ԭ����ƽ�������غ㷨���û�ѧ��������֪ʶ��

 �������������������бز����ٵ���Ҫ��������ͼ����
�������������������бز����ٵ���Ҫ��������ͼ����
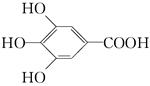 ��ûʳ��������īˮ��Ҫ������________����������(�����)��
��ûʳ��������īˮ��Ҫ������________����������(�����)��