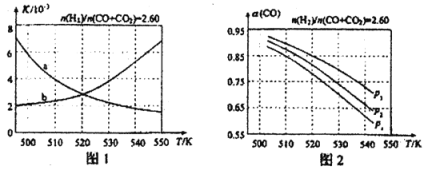
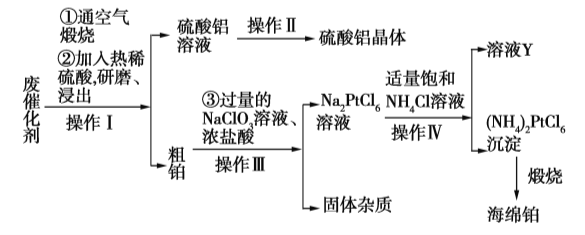
��Ŀ����
����Ŀ��I. ij�л�����C��H��O����Ԫ����ɣ����ģ����ͼ��ʾ��

��1�����л���ķ���ʽ��_____________��
��2�����л�����Է����Ӿ۷�Ӧ�������Ľṹ��ʽ��_____________��
��3�������йظ��л���������У���ȷ����____������ţ���
a. ����NaHCO3��Һ��Ӧ
b. �ܷ���ˮ�ⷴӦ
c. ���������CCl4��Һ��Ӧ
d. �������Ը��������Һ��Ӧ
II. ��1��д�����л���������ƻ�ṹ��ʽ��
��![]() _______________________________��
_______________________________��
��2,5-����-2,4-����ϩ�Ľṹ��ʽ��_______________________��
��2��������ֳƻƼ���ҹ��ض�����ҩ������������е�һ���������ҹ���ѧ���о�������ṹ��ͼ��

������к��������ŵ�������_________________������____________�ࣨ������ӡ�����
���𰸡� C3H4O2 ![]() ad ��������
ad �������� ![]() �ǻ� ��
�ǻ� ��
��������I. �����л�������ģ�Ϳ�֪���л���ṹ��ʽΪCH2=CHCOOH����
��1�����л���ķ���ʽ��C3H4O2����2�����л��ﺬ��̼̼˫�������Է����Ӿ۷�Ӧ�������Ľṹ��ʽ��![]() ����3�������йظ��л���������У���ȷ����____������ţ���
����3�������йظ��л���������У���ȷ����____������ţ���
a. �����Ȼ�������NaHCO3��Һ��Ӧ��a��ȷ��b. ���������������ܷ���ˮ�ⷴӦ��b����c. ����̼̼˫�����������CCl4��Һ��Ӧ��c����d. ����̼̼˫�����������Ը��������Һ��Ӧ��d��ȷ����ѡad��
II. ��1����![]() �����࣬�����Ǽ�����������2��5-����-2��4-����ϩ�Ľṹ��ʽΪ
�����࣬�����Ǽ�����������2��5-����-2��4-����ϩ�Ľṹ��ʽΪ![]() ����2����������صĽṹ��ʽ��֪�����к��������ŵ��������ǻ������ڴ���
����2����������صĽṹ��ʽ��֪�����к��������ŵ��������ǻ������ڴ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�