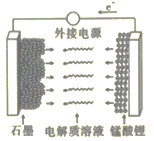
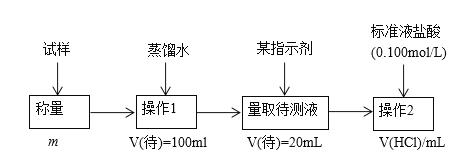
ЬтФПФкШн
ЁОЬтФПЁПЪвЮТЯТЃЌАбЯТСаИїЬтЕФНсЙћЬюдкКсЯпЩЯ
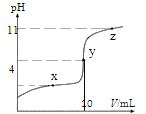
(1)0.0005 mol/LЕФЧтбѕЛЏБЕШмвКЕФpHЃН____________
(2)pHЃН4ЕФHNO3ШмвКжаЃЌЫЎЕчРыГіЕФHЃЋЕФЮяжЪЕФСПЕФХЈЖШc(HЃЋ)ЃН___________molЁЄLЃ1
(3)pHЃН4ЕФNH4ClШмвКжаЃЌЫЎЕчРыГіЕФHЃЋЕФЮяжЪЕФСПЕФХЈЖШc(HЃЋ)ЃН___________mol/L
(4)pHЯрЭЌЕФЯТСаШмвКЃКЂйбЮЫсЁЂЂкДзЫсЃЌЗжБ№гызуСПЧвБэУцЛ§ЯрЭЌЕФаПЦЌЗДгІЃЌЗДгІЦ№ЪМЪБЩњГЩЦјЬхЕФЫйТЪ_______(бЁгУЁАЂйПьЁБЛђЁАЂкПьЁБЛђЁАвЛбљПьЁБЛђЁАЮоЗЈХаЖЯЁБЬюаД)ЃЌзюжеЩњГЩЦјЬхЕФЬхЛ§_______ЁЃ(бЁгУЁАЂйЖрЁБЛђЁАЂкЖрЁБЛђЁАвЛбљЖрЁБЛђЁАЮоЗЈХаЖЯЁБЬюаД)
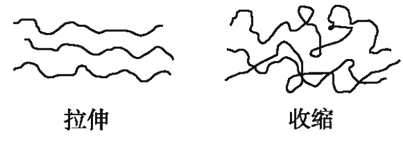
(5)гУРызгЗНГЬЪНБэЪОFeCl3ШмвКЯдЫсадЕФдвђЃК___________
ЁОД№АИЁП11 1ЁС10-10 1ЁС10-4 вЛбљПь ЮоЗЈХаЖЯ ![]()
ЁОНтЮіЁП
ЃЈ1ЃЉ0.0005mol/LЕФЧтбѕЛЏБЕШмвКжаc(OH-)=0.001mol/LЃЌШмвКжа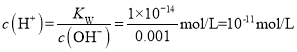 ЃЌШмвКpH=11ЃЛ
ЃЌШмвКpH=11ЃЛ
ЃЈ2ЃЉpHЃН4ЕФHNO3ШмвКжаc(H+)=10-4mol/LЃЌШмвКжа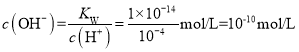 ЃЌШмвКжаOH-ОљЮЊЫЎЕчРыЃЌдђc(H+)ЫЎ= c(OH-)=1ЁС10-10 mol/LЃЛ
ЃЌШмвКжаOH-ОљЮЊЫЎЕчРыЃЌдђc(H+)ЫЎ= c(OH-)=1ЁС10-10 mol/LЃЛ
ЃЈ3ЃЉpHЃН4ЕФNH4ClШмвКжаc(H+)=10-4mol/LЃЌШмвКжаH+ОљЮЊЫЎЫљЕчРыЃЌвђДЫЫЎЕчРыГіЕФHЃЋЕФЮяжЪЕФСПЕФХЈЖШc(HЃЋ)=10-4mol/LЃЛ
ЃЈ4ЃЉЦ№ЪМpHЯрЭЌЃЌвђДЫШмвКжаЧтРызгХЈЖШЯрЭЌЃЌЙЪЗДгІЦ№ЪМЪБЩњГЩЦјЬхЕФЫйТЪЯрЭЌЃЛЮДИцжЊСНШмвКЕФЬхЛ§ЃЌвђДЫВЛФмШЗЖЈзюжеВњЩњЧтЦјЕФСПЖрЩйЃЛ
ЃЈ5ЃЉFeCl3ЮЊЧПЫсШѕМюбЮЃЌЦфЫЎНтРызгЗНГЬЪНЮЊЃК![]() ЁЃ
ЁЃ

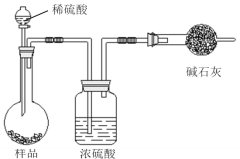
 ЦкФЉМЏНсКХЯЕСаД№АИ
ЦкФЉМЏНсКХЯЕСаД№АИЁОЬтФПЁПгУЭМЫљЪОзАжУМьбщввЯЉЪБВЛашвЊГ§дгЕФЪЧ
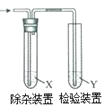
ввЯЉЕФжЦБИ | ЪдМСX | ЪдМСY | |
A | CH3CH2BrгыNaOHввДМШмвКЙВШШ | H2O | KMnO4ЫсадШмвК |
B | CH3CH2BrгыNaOHввДМШмвКЙВШШ | H2O | Br2ЕФCCl4ШмвК |
C | CH3CH2OHгыХЈH2SO4ЙВШШжС170Ёц | NaOHШмвК | KMnO4ЫсадШмвК |
D | CH3CH2OHгыХЈH2SO4ЙВШШжС170Ёц | NaOHШмвК | Br2ЕФCCl4ШмвК |